Case Report
Verrucous Lesions Caused by Disseminated Coccidioidomycosis
Fadi AL Akhrass*, Lina Abdallah, Bharat Ammisetty and Muhannad Antoun
Department of Infectious Diseases and Infectious Control, Pikeville Medical Center, USA
*Corresponding author: Fadi Al Akhrass, Department of Infectious Diseases and Infectious Control, Pikeville Medical Center. 911 Bypass Road, Pikeville KY 41501, USA
Published: 24 Jun, 2017
Cite this article as: Akhrass FAL, Abdallah L, Ammisetty B,
Antoun M. Verrucous Lesions Caused
by Disseminated Coccidioidomycosis.
Ann Clin Case Rep. 2017; 2: 1385.
Abstract
This report describes the case of a young black man in whom disseminated Coccidiodomycosis produced a visually striking facial skin condition. The recognition of the cutaneous signs of diseases that primarily affect the pulmonary system may assist the clinician in diagnosis. Findings on the skin are very helpful in exposing an underlying systemic condition that can be particularly life threatening, and early detection and treatment may impact the course of a patient’s life.
Case Presentation
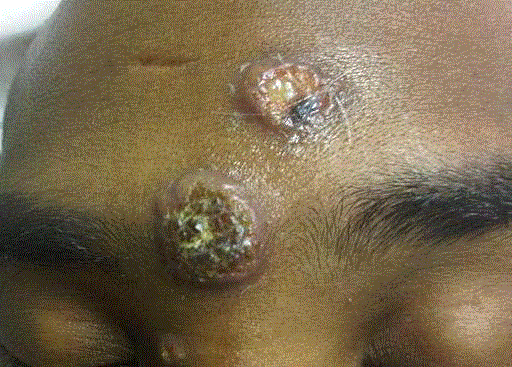
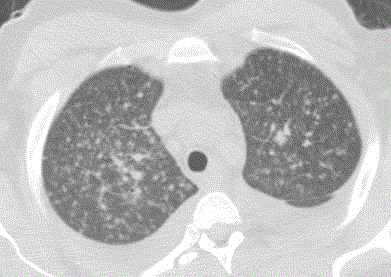
A 34-year-old African American man presented with one-month history of fatigue, diminished appetite, weight loss of 20 lbs, fevers, and cough with purulent sputum production. Two-weeks prior to presentation to the hospital, he developed non-painful and non-pruritic skin lesions over his forehead and nose. The patient visited California almost 11 months before the appearance of his clinical manifestations. Examination revealed a high-grade fever of 105oF, hypoxemia with oxygen saturation of 87% without supplemental oxygen, and tachycardia of 117 beats per minute. The patient was confused, with slow speech, but neurological examination was non focal. His lung exam revealed diffuse bilateral wheezes. The skin lesions had progressed to verrucous nodular lesions on his face (forehead and nose) (Figure 1). Laboratory testing revealed a white blood cell count of 6500 cells/mm3 (10% eosinophils),), LDH levels of 636 IU/L, ALT of 102 IU/L, creatinine of 2.3 mg/dL and BUN of 60 mg/dL. A computerized tomography scan demonstrated innumerable military nodules with associated interstitial thickening with hilar and sub clavian lymphadenopathies (Figure 2). Magnetic resonance of the brain showed advanced cortical atrophy. Bronchoscopy revealed multiple necrotic lesions at the main carina and its bifurcation. Serum Coccidioides, Bastomyces and Histoplasma serologies, urine Histoplasma antigen assay and tuberculous skin tests were negative. His HIV-1 test came back positive with CD4 cell count of 8 cells/mm3 and a viral load of 490,000 copies/ml.
Diagnosis
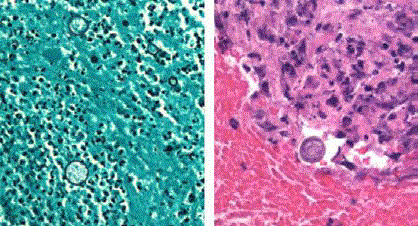
Spherules of Coccidioides were identified on a skin and transbronchial biopsy samples, and tissue cultures grew Coccidioides immitis after 4 days (Figures 3A and B). The fungus was also isolated from the blood cultures. Disseminated Coccidiodomycosis was diagnosed.
Diagnosis
Coccidioidomycosis is well known for its ability to masquerade as other infectious and noninfectious
disease processes. Disseminated cutaneous coccidioidomycosis should be considered in the
differential diagnosis of any chronic nodular or verrucous skin lesions, especially when associated
with pulmonary manifestations.
Coccidioidomycosis is caused by the highly infectious dimorphic fungi of the genus Coccidioides
(C. immitis and C. posadasii) [1]. Coccidioides species are endemic to desert regions of the Southwestern United States, northern Mexico, and scattered areas in Central America and South
America [2]. However, infections have been reported outside these endemic areas [3]. In fact, travel
plays a major role in the diagnosis of different fungal infections and even a short exposure history
should not be ignored. The use of potent antiretroviral therapy (ART), which results in immune
restoration and increased CD4 cell count, appears to have a marked effect on both the incidence and
the severity of symptomatic coccidioidomycosis [4,5]. In one study, almost one half of the cases of coccidioidomycosis in AIDS patients were from non-endemic areas of the United States [6]. Thus,
reactivation of latent infection should be suspected in patients diagnosed with coccidioidomycosis
who have not recently been in the area of endemicity [6,7,8]. Coccidioides infection is always acquired by inhalation of soil or dust containing the fungus. Within the lung,
an arthroconidium changes from a barrel-shaped cell to a spherical
structure called spherule. Enlarging spherules produce internal
septations, and within each of the resulting sub compartments
individual cells (endospores) evolve. After several days, mature
spherules rupture releasing endospores into the infected tissues; each
endospore can produce another spherule [1]. Coccidioidomycosis
commonly spreads from the thoracic cavity to other parts of the
body and can cause lymphatic, meningeal, mucocutaneous, and
musculoskeletal involvement [9]. Cutaneous presentations may be
divided into reactive or organism-specific reactions. Patients with
primary pulmonary infection may present with reactive erythema
nodosum, erythema multiforme, sweet’s syndrome, or a generalized
morbilliform rash. Organism-specific cutaneous involvement is
generally secondary to hematogenously disseminated disease but
can rarely represent primary disease from direct inoculation [10].
Disseminated cutaneous Slesions are typically localized to the head
and neck. These lesions vary widely in appearance from papules,
nodules, plaques, ulcerations, and abscesses to lesions that imitate
T-cell lymphoma mycosis fungoides [10]. Primary cutaneous
infections are often self-limited and heal within weeks [11]. They
present as ulcerated nodules at the site of inoculation with associated
lymphadenopathy with further development of nodules along the
draining lymphatic tracts. Diagnostic criteria to distinguish primary
cutaneous disease include an absence of pulmonary involvement,
evidence of traumatic inoculation of skin site, incubation of 1–3
weeks before visible lesion, and low level complement fixation titers
[11].
Figure 1
Figure 1
Two verrucous lesions aspect located on the forehead (1,2x2 cm
and 3x3 cm) and 1 small nodule (0.6x0.5 cm) on the medial side of the nasal
bridge.
Figure 2
Figure 2
Computed Tomography axial cut of the chest demonstrated
innumerable miliary nodules with associated interstitial thickening with hilar
and subclavian lymphadenopathies.
Figure 3
Figure 3
Skin biopsy section stained with Giemsa (A) and lung biopsy
section stained with hemotoxylin and eosin (B) (original magnification, ×200)
demonstrated spherules of Coccidioides immitis.
Management
Given the acuity, extent and severity of our patient’s disease,
therapy was promptly initiated with systemic amphotericin B
deoxycholate and fluconazole. Disseminated coccidioidomycosis
may progress to severe disease and a potentially fatal outcome,
thus warranting timely recognition and accurate management. The
Infectious Disease Society of America published in 2005 detailed
guidelines on the treatment of systemic coccidioidomycosis [3].
Symptomatic disseminated disease requires immediate treatment
with Amphotericin B or an azole antifungal. After initial treatment of
the disseminated infection, maintenance therapy for 1 to 2 years or
life-long therapy may be required. Because the critical factor in the
control of coccidioidomycosis is cellular immune function, effective
ART was instituted concomitantly with initiation of antifungal
therapy. Immune response inflammatory syndrome (IRIS) has
been observed in HIV-infected patients with a variety of underlying
infections as they respond to potent antiretroviral therapy [12].
Fortunately, our patient demonstrated a fast response to medical
therapy. Respiratory, cutaneous, and systemic manifestations as well
as radiographic abnormalities had markedly improved. Findings on
the skin were very helpful in exposing an underlying life threatening
systemic condition, leading to early detection and treatment that had
positively impacted the course of our patient’s life.
Authorship Statement
All work in this manuscript is original. All authors had access to the data and played a role in writing the manuscript and each accepts responsibility for the content.
References
- Saubolle MA, McKellar PP, Sussland D. Epidemiologic, clinical, and diagnostic aspects of coccidioidomycosis. J Clin Microbiol. 2007; 45: 26-30.
- Chu JH, Feudtner C, Heydon K, Walsh TJ, Zaoutis TE. Hospitalizations for endemic mycoses: a population-based national study. Clin Infect Dis. 2006; 42: 822-25.
- Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Johnson RH, Stevens DA, et al. Coccidioidomycosis. Clin Infect Dis. 2005; 41:1217-23.
- Woods CW, McRill C, Plikaytis BD, N E Rosenstein, D Mosley, D Boyd, et al. Coccidioidomycosis in human immunodeficiency virus-infected persons in Arizona, 1994-1997: incidence, risk factors, and prevention. J Infect Dis. 2000; 181:1428-34.
- Masannat FY, Ampel NM. Coccidioidomycosis in patients with HIV-1 infection in the era of potent antiretroviral therapy. Clin Infect Dis. 2010; 50:1-7.
- Jones JL, Fleming PL, Ciesielski CA, Dale J Hu, Jonathan E, Kaplan, et al. Coccidioidomycosis among persons with AIDS in the United States. J Infect Dis. 1995; 171: 961-66.
- Forseth J, Rohwedder JJ, Levine BE, Saubolle MA. Experience with needle biopsy for coccidioidal lung nodules. Arch Intern Med. 1986; 146: 319-20.
- Hernández JL, Echevarría S, García-Valtuille A, Mazorra F, Salesa R. A typical coccidioidomycosis in an AIDS patient successfully treated with fluconazole. Eur J Clin Microbiol Infect Dis. 1997; 16: 592-94.
- Ampel NM. Coccidioidomycosis in persons infected with HIV type 1. Clin Infect Dis. 2005; 41: 1174-1178.
- Crum NF. Disseminated coccidioidomycosis with cutaneous lesions clinically mimicking mycosis fungoides. Int J Dermatol. 2005; 44: 958-960.
- Chang A, Tung RC, McGillis TS, Bergfeld WF, Taylor JS. Primary cutaneous coccidioidomycosis. J Am Acad Dermatol. 2003; 49: 944-949.
- DeSimone JA, Pomerantz RJ, Babinchak TJ. Inflammatory reactions in HIV-1-infected persons after initiation of highly active antiretroviral therapy. Ann Intern Med. 2000; 133: 447-454.