Case Report
Favourable Outcome of Two Contraindicated Pregnancies in a Woman with Complex Congenital Heart Disease and Eisenmenger Syndrome
Dauphin C1*, Laurichesse H1, Storm B1 and Lusson JR2
1Department of Cardiology and Vascular Diseases, CHU Gabriel-Montpied, France
2Department of Rheumatology, CHU Gabriel-Montpied, France
*Corresponding author: Claire Dauphin, Department of Cardiology and Vascular Diseases, CHU Gabriel-Montpied, Clermont-Ferrand, F-63003, France
Published: 05 Jun, 2017
Cite this article as: Dauphin C, Laurichesse H, Storm B,
Lusson JR. Favourable Outcome of
Two Contraindicated Pregnancies in a
Woman with Complex Congenital Heart
Disease and Eisenmenger Syndrome.
Ann Clin Case Rep. 2017; 2: 1366.
Abstract
We report the case of a young woman who was born with complex heart disease consisting in a single ventricle, double discordance, tricuspid atresia without pulmonary obstacle, and fixed Pulmonary
Arterial Hypertension (PAH). She carried two pregnancies to term against medical advice after
percutaneous dilatation of a restrictive atrial septal defect.
The good functional tolerance of her heart disease (class I-II NYHA), saturation greater than 85%,
and early multi-disciplinary management of the pregnancies undoubtedly contributed to their
successful outcome.
Our patient’s experience should not call into question the contraindication for pregnancy in this
type of disease but may help in the management of similar women who, although aware of the risk,
desire to become pregnant.
Introduction
The prognosis of Eisenmenger Syndrome (ES) is usually better than that of idiopathic Pulmonary Hypertension (PAH) [1]. However, the more complex the underlying heart disease, the less favourable it becomes [2]. Pregnancy carries a high risk of mortality for women with ES and is therefore strictly contraindicated [3-6].
Case Presentation
We report the case of a young woman who was born with complex heart disease consisting
in a single ventricle, double discordance, tricuspid atresia without pulmonary obstacle, and fixed
Pulmonary Arterial Hypertension (PAH) who became pregnant against medical advice and carried
two uneventful pregnancies successfully to term.
Mrs P. was born 3/4/1980. The precise diagnosis of her heart condition was made by
ultrasonography when she was 8 years old following pneumonia. Her only extra-cardiac history
was scoliosis, which was operated on under loco-regional anaesthesia at the age of 14 years. Her
clinical status remained stable throughout adolescence with stage III dyspnoea, saturation between
75 and 80% and polycythemia (haemoglobin level of 20.5 g/l), and she was able to work part-time as
a secretary. In November 2004, she was admitted to hospital for worsening of her dyspnoea during
a period of bronchial superinfection. A diagnosis of pulmonary oedema was suspected on the basis
of combined cough, orthopnoea and disclosure of bilateral crepitations. Chest x-ray showed diffuse
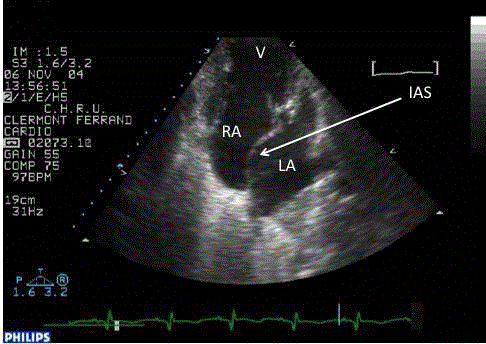
interstitial syndrome, and ultrasonography restrictive Atrial Septal Defect (ASD) (Figure 1). The patient improved on diuretics and antibiotics.
It was decided to dilate her ASD by interventional catheterism under Transoesophageal
Echocardiographic (TEE) guidance. TEE confirmed diagnosis, showing spontaneous contrast in the
left atrium, which was very “stretched” and measuring a continuous gradient through the ASD of
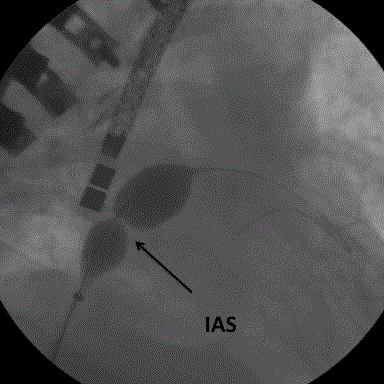
16 mmHg. PAH was systemic (Table 1) with elevated capillary pressure. The ASD was dilated with a balloon 25 mm in diameter (Figure 2). At the end of the procedure, left atrial pressure fell to the level of that of the right atrium but PAH remained systemic (Table 1, Figures 3 and 4).
The patient’s functional improvement was dramatic (class I-II) and she was able to walk up four
flights of stairs, and put on weight (+7kg). She began considering the possibility of conceiving a child. Saturation was 85%.
Despite repeated reminders of the contraindications, Mrs. P
became pregnant. She presented in September 2006 in her seventh
week of pregnancy with saturation 92% and a haemoglobin level of 16.5
g/dl. The couple were once again warned that the pregnancy exposed
mother and unborn child to risks but they decided nevertheless to go
ahead. A cardiological examination was performed every 4 to 6 weeks
and a multidisciplinary team comprising anaesthetists, cardiologists
and obstetricians discussed how the birth should be managed in the
event of an emergency. All decisions arrived at were recorded in the
patient’s file. The patient’s pelvis was narrow and so it was decided to
perform a caesarian section, before term but if possible after 32 weeks,
under continuous spinal anaesthesia with invasive monitoring of
blood pressure. During pregnancy, her clinical status was stable with
saturation greater than 85% and she gave birth to a boy weighing 1 kg
945 (APGAR 8-10-10) at 34wk+4d. She was monitored for 48h in the
cardiology intensive care unit and then 5 days in the maternity unit.
The child stayed 1 month in the neonatology department. Treatment
with Low-Molecular-Weight Heparin (LMWH) at a curative dose
was prescribed, later replaced by Anti-Vitamin K (AVK). Diuretics
were reintroduced distance to birth owing to left- and rightcongestive
signs, and Converting Enzyme Inhibitor (CEI) treatment
was initiated in November 2008 of systematic way (single ventricle and slight leakage from the right mitral valve).
Mrs. P. became pregnant a second time. She presented in
February 2012 at 5 weeks of amenorrhoea with the firm intention of
continuing the pregnancy despite reiteration of the risks involved. On
her own initiative, she stopped CEI treatment. AVK were replaced by
LMWH during the first term. The pregnancy was managed under the
same conditions as the first. She underwent caesarian section at 35
weeks of amenorrhoea after corticosteroid treatment to accelerate
fetal lung maturation and replacement of AVK by LMWH, under
continuous spinal anaesthesia. At the patient’s request, perioperative
tubal ligation was performed. She gave birth to a girl weighing 2 kg
030 (APGAR 3, 6, 8), who was admitted to paediatric intensive care
for respiratory distress. Postpartum progress was uneventful except
for marked asthenia, which delayed discharge until day 16.
Table 1
Figure 1
Figure 2
Figure 3
Figure 4
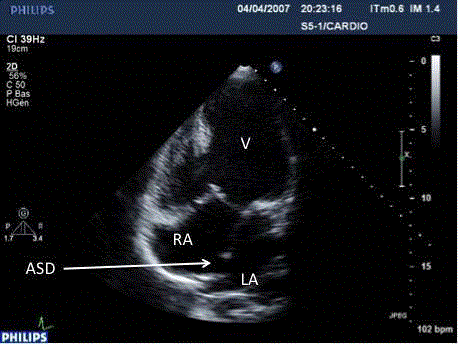
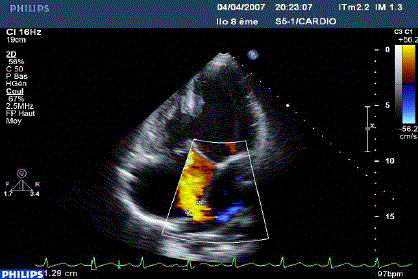
Figure 4
TTE: Apical view, colour Doppler: non-restrictive left-right flow,
through the ASD after dilatation of the IAS.
Discussion
In recent years, the number of adults suffering from complex
congenital heart disease has continued to rise owing to improved
management of the condition. Marelli [7] reported a prevalence of
0.38 per1000 adults, with a female predominance (57%). In developed
countries, maternal heart disease has become the leading cause of
death during pregnancy [8], and congenital heart disease the most
frequently encountered heart disease in pregnant women [9].
Such patients should be monitored at least once a year at a
“GUCH” specialised centre [4]. In the case of our patient, this followup
led to a reassessment of her condition, which until then had been
considered as stable and intractable to treatment, and the decision to
perform percutaneous dilatation of her atrial septum, which although
only a palliative intervention transformed her functional status. The recommendations concerning pregnancy in patients with a single ventricle are clear: “Pregnancy is contraindicated in cases of severely
restricted pulmonary airflow, severe pulmonary vascular disease
(Eisenmenger syndrome) and poor ventricular function” [4]. The
only element in our patient’s favour before pregnancy was her class
II NYHA [3].
PAH is an absolute contraindication for pregnancy, with a
reported maternal mortality rate of 30 to 50% in earlier series
[10], and one of 17 to 33% in more recent publications [11]. Only patients with positive long-term response to calcium channel
blockers seem to have a more favourable prognosis [12]. The risk
of maternal death occurs during the last trimester of pregnancy
or in the first weeks postpartum owing to PAH attack, pulmonary
thrombosis or intractable right heart failure. Eisenmenger syndrome
[3-5] compounds the risks of PAH and right-left shunt, which can
worsen during pregnancy as the result of decreased systemic vascular
resistance. A recent publication reports a cardiac morbidity of 33%
and a mortality of 5% in a French multi-center series of 20 patients (28
pregnancies) [13]. If the pregnancy is continued, rest, anticoagulation
therapy, (at least preventive regimen) and controlled intake of iron
substitutes are recommended [14]. Oxygen saturation and blood
counts should be regularly monitored. Owing to haemodilution,
the level of haemoglobin is less reliable at the end of pregnancy than
saturation [15].
Maternal cyanosis is itself a serious risk factor during pregnancy,
in particular for the fetus. In a series of 96 pregnancies in 44 patients
with cyanotic heart disease, excluding those with Eisenmenger
syndrome, Presbitero et al. [16] reported a maternal morbidity rate
of 32% and only one death but a high rate of fetal complications:
premature birth (37%), hypotrophy, miscarriage, and fetal death in
utero. The percentage of live births was directly related to the extent
of hypoxia (12% of live births when saturation was lower than 85%,
45% from 85 to 90%, and 92% when it was higher than or equal to
90%), to the level of haemoglobin ( 71% of live births when it was
lower than or equal to 16 g/dl, 45% between 17 and 19 g/dl and 8%
when it was higher than or equal to 20 g/dl), and to the type of heart
disease (31% of live births in the “single ventricle” group).
Cardiac failure can be prevented by rest and early administration
of diuretics, at the lowest dose possible to avoid haemoconcentration.
Because of the occurrence of endocarditis postpartum in 7% of the
patients (one of whom died 2 months after delivery), the authors
consider prophylactic antibiotic treatment to be mandatory during
delivery.
Management of delivery [6] should be discussed for each
individual case according to the patient and the practice of the
medical team. Swan Ganz catheterisation is not advisable since the
risk of complications outweighs the potential benefits [3]. The risks
of becoming pregnant should be explained early on to young women
with severe heart disease, re-explained at regular intervals and once
again in the event of pregnancy. Termination of the pregnancy can be
envisaged because of the threat to the mother’s health but itself is not
without risk [6].
In their article Kovacs et al. [15] questioned women with
congenital heart disease to see whether their physician had informed
them of the risks involved in becoming pregnant. Of the 80 women
at intermediate to high risk, 34 % had received no information, and
out of 18 women for whom pregnancy was contraindicated, 9 had
not been warned of the potential harm. Presbitero et al. [16] observed
functional impairment in their patient series during the 2 years
following delivery but were unable to conclude whether it was due
to the natural progress of the disease or to the additional workload of
looking after an infant. This possibility should be discussed with the
couple before or during the pregnancy and social assistance should be
systematically offered during the postpartum period.
Conclusion
Improved management of congenital heart disease has led to an increase in the number of women of childbearing age suffering from the condition. Cyanotic heart disease, particularly in combination with PAH, such as Eisenmenger syndrome, still remains a contraindication for pregnancy because of the high risk of maternal mortality. Although informed of the risk to life involved in becoming pregnant, certain women decide nevertheless to try to conceive. Early and multidisciplinary management of the pregnancy in a specialized center should be undertaken to ensure mother and child every chance of successful outcome.
References
- Hopkins WE, Ochoa LL, Richardson GW, Trulock EP. Comparison of the hemodynamics and survival of adults with severe primary pulmonary hypertension or Eisenmenger syndrome. J Heart Lung Transplant. 1996; 15: 100-105.
- Diller GP, Dimopoulos K, Broberg CS, Kaya MG, Naghotra US, Uebing A, et al. Presentation, survival prospects, and predictors of death in Eisenmenger syndrome: a combined retrospective and case-control study. Eur Heart J. 2006; 27: 1737-1742.
- Regitz-Zagrosek V. ESC Guidelines on the management of cardiovascular diseases during pregnancy. Eur Heart J. 2011; 32: 3147-3197.
- Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, et al. ESC Committee for Practice Guidelines (CPG). ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J. 2010; 23: 2915-2957.
- Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009; 34:1219-1263.
- Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, et al. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines for the management of adults with congenital heart disease). Circulation. 2008; 118: 2395-2451.
- Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007; 115: 163-172.
- Cantwell R, Clutton-Brock T, Cooper G, Dawson A, Drife J, Garrod D, et al. Saving Mothers' Lives: Reviewing maternal deaths to make motherhood safer: 2006-2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011; 118: 1-203.
- Siu SC. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation 2001. 104: 515-521.
- Weiss BM, Zemp L, Seifert B, Hess OM. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996. J Am Coll Cardiol. 1998; 31: 1650-1657.
- Bédard E, Dimopoulos K, Gatzoulis MA. Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension?. Eur Heart J. 2009; 30: 256-265.
- Jaïs X, Olsson KM, Barbera JA, Blanco I, Torbicki A, Peacock A, et al. Pregnancy outcomes in pulmonary arterial hypertension in the modern management era. Eur Respir J. 2012; 40: 881-885.
- Ladouceur M, Benoit L, Radojevic J, Basquin A, Dauphin C, Hascoet S, et al. Pregnancy outcomes in patients with pulmonary arterial hypertension associated with congenital heart disease. Heart. 2016; 103: 287-292.
- Avila WS, Maternal and fetal outcome in pregnant women with Eisenmenger’s syndrome. Eur Heart J. 1995; 16: 460-464.
- Kovacs AH, Harrisson JL, Colman JM, Sermer M, Siu SC, Silversides CK. Pregnancy and contraception in congenital heart disease: What women are not told. J Am Coll Cardiol. 2008; 52: 577-578.
- Presbitero P, Somerville J, Stone S, Aruta E, Spiegelhalter D, Rabajoli F. Pregnancy in cyanotic congenital heart disease. Outcome of mother and fetus. Circulation. 1994; 89: 2673-2676.