Case Report
Effect of Cooling Intervention on Reducing Rigidity in Parkinson’s Disease: A Case Report
RuiPing Xia*, Caleb Gillig, Drew Mueller, Mitchell Ludwig and Zachary Schmith
Department of Physical Therapy, University of St. Mary, USA
*Corresponding author: RuiPing Xia, Department of Physical Therapy, University of St. Mary, 4100 S. 4th Street, Leavenworth KS, USA
Published: 31 May, 2017
Cite this article as: RuiPing Xia, Gillig C, Mueller D,
Ludwig M, Schmith Z. Effect of Cooling
Intervention on Reducing Rigidity in
Parkinson’s Disease: A Case Report.
Ann Clin Case Rep. 2017; 2: 1363.
Abstract
Objective: Describe potential changes in parkinsonian rigidity after an administration of cooling intervention.
Methods: Two patients with Parkinson’s Disease (PD) participated in the study that applied the
forearm cooling intervention and objectively evaluated rigidity at the wrist joint through a cycle of
passive flexion and extension movements of ±45° at a velocity of 50°/sec. Both patients underwent
a baseline assessment (Pretest), and an immediate assessment after cooling intervention (Posttest)
and 30 minutes after the cooling (30-min Posttest). Rigidity temporal score (Nm-sec) and work
score (Nm-deg) were used to quantify and compare rigidity between the Pretest and the Posttests.
Results: In Case #1, there were reductions in both rigidity scores at Posttest and relatively smaller
reductions at 30-min Posttest after cooling. Case 2 demonstrated a slight increase immediately
posttest which was followed with a slight decrease at 30-min Posttest.
Discussion and Conclusion: Cooling intervention appears to be a promising therapy in treating
parkinsonian rigidity. Future research on a larger sample size is warranted for further investigation
of the effectiveness of cooling therapy.
Keywords: Parkinson’s disease; Rigidity; Physical therapy; Cooling intervention
Introduction
Parkinson’s Disease (PD) is a chronic, progressive neurodegenerative disease that is
characterized by both motor and nonmotor symptoms including but not limited to bradykinesia,
rigidity, cognitive impairments, and fatigue [1,2]. As the disease advances, PD symptoms become more severe. Rigidity progresses more relentlessly than other cardinal symptoms [3-5]. Rigidity is defined as an increased resistance to passive movement of a limb persisting throughout its range of
motion, also referred to as hypertonia [6]. Many studies have shown that patients with PD exhibit a marked increase in long-latency stretch reflexes, compared to healthy controls [7-10]. Exaggerated stretch reflexes are the primary mechanism underlying parkinsonian rigidity.
Research has suggested the beneficial effects of rehabilitation programs, such as physical
therapy, on the treatment of PD [11,12]. But the effect of physical therapy on reducing rigidity in PD remains unknown. Nevertheless, there is a body of evidence on the effectiveness of physical
therapy treatment in managing spasticity. Rigidity and spasticity share common clinical signs, and
both are characterized by hypertonia [9,10]. Cooling therapy (or cryotherapy) has been widely used to manage spasticity in clinical practice with its efficacy judged mainly by clinical assessments.
Furthermore, numerous studies have demonstrated positive effect of cooling on reducing spasticity
in upper motor neuron syndromes [13-17].
None of the study has examined the effect of cooling intervention on rigidity. Based upon
evidence that cooling is effective in decreasing spasticity, we hypothesized that rigidity could
potentially be decreased by cooling therapy. The purpose of this case report was to describe the
effects of the cooling intervention on rigidity at the wrist joint in two subjects with PD.
Case Presentation
Participants
Two patients with idiopathic PD participated in this study. One was a 76-year-old man with
disease duration of 2 years, and the other was a 70-year-old woman with disease duration of 7 years.
The Hoehn and Yahr stages were 2.5 and 2, respectively. In both cases, the left side exhibited greater rigidity. Both were instructed not to alter their medication routine
during the participation. The experimental protocol was approved
by the Institutional Review Board of the University of St. Mary in
Leavenworth, Kansa, USA. Informed consent was obtained from each
patient prior to the participation.
Protocol
The procedures included the Motor Examination of the Unified
Parkinson’s Disease Rating Scale (UPDRS) [18] and then an objective
assessment of rigidity [19-23]. For objective assessments, each patient
was seated in a height-adjustable chair with the left hand being held
in a manipulandum. The elbow was in approximately 120° of flexion
with the wrist and forearm in neutral. Each patient was instructed
to remain relaxed during passive wrist flexion and extension
movements generated by the servomotor. Each trial began with the
wrist at approximately 45° of wrist extension and moved through a
central range of motion of 90° (±45°) at a constant velocity of 50°/sec.
Details on the objective assessment of rigidity have been described
previously [19-23].
After the baseline assessment (Pretest), a cooling intervention
was administered, and then followed by an immediate assessment
of rigidity (Posttest) and subsequently a 30-minute post-cooling
assessment (30-min Posttest).
Cooling intervention
A cooling garment was utilized and applied to implement the
cooling intervention. The initial temperatures in the skin near elbows
of the tested limbs were 30.4°C and 31.7°C for the two patients,
respectively. The left forearm of each participant was wrapped in
the garment with embedded fluid channels supplied with circulating
fluid by a commercial chiller (Polar Products, Inc., OH), until the
skin temperature near the elbow that was concurrently monitored by
a traceable digital thermometer with a probe (Fisher Scientific, Co.,
NH) cooled to 25°C. The cooling continued for additional 15 minutes.
At the end of this duration, the cooling garment was then removed from subjects’ forearms. Immediately, the objective assessment of
rigidity was performed (Posttest), and performed again 30 minutes
after the removal of the garment (30-min Posttest).
Outcome measures and data analyses
Joint torque and angular position about the wrist were recorded
from each participant. Torque was quantified by integrating the
rectified torque with respect to time (i.e., “temporal or angular score”
in Nm-sec) and by integrating the non-rectified torque with respect to
joint angle (i.e., “work score” in Nm-deg). The procedures provided
objective quantifications of torque resistance, yielding two forms of
objective rigidity scores [19,24]. The angular score has been shown to
be a valid objective measure of rigidity that improves the sensitivity
and reproducibility of assessment [24]. The higher the objective
rigidity scores were, the more severe the rigidity was.
Figure 1
Figure 1
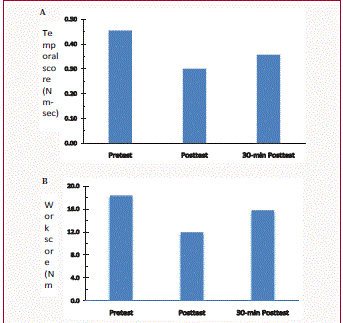
Rigidity scores measured at three time points for Case #1: before
cooling therapy (Pretest), immediately after cooling therapy (Posttest), and
30-min after cooling (30-min Posttest). A: Temporal score; B: Work score.
Figure 2
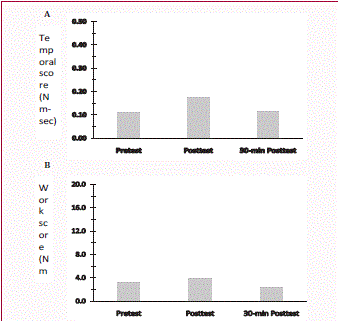
Figure 2
Rigidity scores measured at three time points for Case #2: before
cooling therapy (Pretest), immediately after cooling therapy (Posttest), and
30-min after cooling (30-min Posttest). A: Temporal score; B: Work score.
Results and Discussion
The baseline temporal and work scores for Case #1 were 0.45
Nm-sec and 18.32 Nm-deg (Figure 1). Immediately after cooling
intervention, rigidity scores decreased to 0.30 Nm-sec for temporal
score and to 11.93 Nm-deg for work score, respectively. At 30-min
Posttest, rigidity scores showed increases to 0.36 Nm-sec and 15.77
Nm-deg, compared to Posttest. However, the scores remained
smaller than the Pretest measures (Figure 1). In Case #2, baseline
scores were 0.11 Nm-sec and 3.13 Nm-deg. Slight increases were
observed for temporal score (0.17 Nm-sec) and work score (3.88 Nmdeg)
at Posttest measures. There were reductions for both temporal
and work scores (0.11 Nm-sec and 2.41 Nm-deg) at 30-min Posttest
(Figure 2). The work score at 30-min Posttest was lower than that of
Pretest (Figure 2B).
The present study reported potentially beneficial effects of cooling
intervention on managing rigidity in PD. While the concept of cooling
interventions is not completely novel to the realm of physical therapy,
and previous studies have examined the effects of cooling on reducing
spasticity, its application in treating parkinsonian rigidity has never been studied. The current case report is the first study describing the effect of cooling in managing parkinsonian rigidity.
Several studies have shown that rigidity is attributed to
exaggerated long-latency stretch reflexes [7-10]. It has been reported
that cooling therapy (or cryotherapy) can decrease the sensitivity of
muscle spindles to stretch, and alter the stretch reflexes in spasticity
[13-15,25]. In addition, there has been long-standing reports that
when nerve temperature is decreased, nerve conduction velocity is
decreased following cryotherapy [26,27].
Results obtained from this report showed that Case #2 appeared
to be less responsive to cooling intervention. During participation,
patients were instructed not to change the schedule for dopaminergic
medication. It is noted that it generally takes about 30 minutes for
dopaminergic medication to take an effect, and reach its peak level
approximately 60 minutes after taking medicine. Also, the timeline
for medication effect can vary from one to another patient. In this
report, the baseline or pretest measures for the two patients were
assessed 60 and 85 minutes post-medication, respectively. Medication
is expected to influence the study findings. It is highly likely that
medication might have diminished the therapeutic effect of cooling
intervention on rigidity. On the other hand, this may explain the
differences observed between the two patients.
It is acknowledged that the study has limitations. Results were
based on a small number of participants. Case reports cannot establish
any causal relationship. Further, the protocol was performed in the
state of On-medication that can potentially confound the outcomes
of the study.
Conclusion
This case reports provided preliminary results with respect to the potential benefits of cooling for parkinsonian rigidity. The study is of novelty since there has been no previous study that has examined the efficacy of cooling intervention in this patient population. Further, this report is clinically significant, because rigidity impairs patients’ motor function and quality of life by limiting mobility and impeding balance and gait [28,29]. A temporary 30-min reduction in rigidity provides a practical and realistic duration for patients to participate in physical activity and exercise. Accumulating evidence indicates that exercises are beneficial to slowing disease progression and improving function and quality of life in patients with Parkinson’s disease. Cryotherapy can be applied with an ease of use and minimal side effect. Future study may be conducted to incorporate a temporary withdrawal of medication (i.e., Off-medication) to eliminate any confounding influence. Future research utilizing an experimental design performed on a larger sample size of patients and comparison control group is warranted for further investigation on this topic.
Acknowledgement
The authors would like to thank Ms. Melinda Van Velzer for her assistance throughout the project. No conflicts of interest exist between the authors and any outside organization.
References
- Fahn S. Parkinson's disease: 10 years of progress, 1997-2007. Mov Disord. 2010; 25: S2-S14.
- Chaudhuri KR, Naidu Y. Early Parkinson’s disease and non-motor issues. J Neurol. 2008; 255: 33-38.
- Dorschner J, Farmakis G, Behnke S, Hellwig D, Schneider S, Fassbender K, et al. Myocardial MIBG scintigraphy may predict the course of motor symptoms in Parkinson’s disease. Park Relat Disord. 2011; 17: 372-375.
- Rossi C, Frosini D, Volterrani D, De Feo P, Unti E, Nicoletti V, et al. Differences in nigro-striatal impairment in clinical variants of early Parkinson’s disease: evidence from a FP-CIT SPECT study. Eur J Neurol. 2010; 17: 626-630.
- Xia R, Mao Z. Progression of motor symptoms in Parkinson’s disease. Neurosci Bull. 2012; 117: 2302-2307.
- Hallett M. Parkinson revisited: pathophysiology of motor signs. Adv Neurol. 2003; 91: 19-28.
- Lee RG, Tatton WG. Motor responses to sudden limb displacements in primates with specific CNS lesions and in human patients with motor system disorders. Can J Neurol Sci. 1975; 2: 285-293.
- Rothwell JC, Obeso JA, Traub MM, Marsden CD. The behaviour of the long-latency stretch reflex in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1983; 46: 35-44.
- Fung V, Thompson P. Rigidity and Spasticity. Vol 4th ed. Philadelphia: Lippencott Williams & Wilkens; 2002.
- Xia R. Physiological and biomechanical analyses of rigidity in Parkinson’s disease. In: Rana AQ, ed. Etiology and Pathophysiology of Parkinson’s Disease. Vol Vienna, Austria. 2011: 485-506.
- King LA, Horak FB. Delaying mobility disability in people with Parkinson disease using a sensorimotor agility exercise program. Phys Ther. 2009; 89: 384-393.
- Carpinella I, Cattaneo D, Bonora G, Bowman T, Martina L, Montesano A, et al. Wearable sensor-based biofeedback training for balance and gait in Parkinson disease: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2017; 98: 622-630.e3.
- Knuttsson E. Topical cryotherapy in spasticity. Scand J Rehabil Med. 1970; 2: 159-163.
- Price R, Lehmann JF, Boswell-Bessette S, Burleigh A, deLateur BJ. Influence of cryotherapy on spasticity at the human ankle. Arch Phys Med Rehabil. 1993; 74: 300-304.
- Harlaar J, Ten Kate JJ, Prevo a J, Vogelaar TW, Lankhorst GJ. The effect of cooling on muscle co-ordination in spasticity: assessment with the repetitive movement test. Disabil Rehabil. 2001; 2311: 453-461.
- Abd El-Maksoud GM, Sharaf GM, Rzk-Allah SS. Efficacy of cold therapy on spasticity and hand function in children with cerebral palsy. J Adv Res. 2011; 2: 319-325.
- Avilala SD, Kumary S. A comparative study on the efficacy of cryostretches versus hold-relax on plantar flexors spasticity in the subjects with stroke. Indian J Physiother Occu Ther. 2013; 7: 211-218.
- Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society UPDRS Revision Task Force. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008; 23: 2129-2170.
- Xia R, Markopoulou K, Puumala SE, Rymer WZ. A comparison of the effects of imposed extension and flexion movements on Parkinsonian rigidity. Clin Neurophysiol. 2006; 117: 2302-2307.
- Xia R, Sun J, Threlkeld AJ. Analysis of interactive effect of stretch reflex and shortening reaction on rigidity in Parkinson's disease. Clin Neurophysiol. 2009; 120: 1400-1407.
- Powell D, Hanson N, Threlkeld AJ, Fang X, Xia R. Enhancement of parkinsonian rigidity with contralateral hand activation. Clin Neurophysiol. 2011; 122: 1595-1601.
- Powell D, Threlkeld AJ, Xiang Fang, Muthumani A, Xia R. Amplitude- and velocity-dependency of rigidity measured at the wrist in Parkinson’s disease. Clin Neurophysiol. 2012; 123: 764-773.
- Powell DW, Muthumani A, Xia R. A comparison of the effects of continuous versus discontinuous movement patterns on parkinsonian rigidity and reflex responses to passive stretch and shortening. J Nat Sci. 2016; 2: e201.
- Fung V, Burne J, Morris J. Objective quantification of resting and activated parkinsonian rigidity: a comparison of angular impulse and work scores. Mov Disord. 2000; 15: 48-55.
- Bell KR, Lehmann JF. Effect of cooling on H- and T-reflexes in normal subjects. Arch Phys Med Rehabil. 1987; 68: 490-493.
- Lee J, Warren M, Mason S. Effects of ice on nerve conduction velocity of the ulnar nerve. Physiotherapy. 1978; 64: 2-6.
- Zankel H. Effect of physical agents on motor conduction velocity of the ulnar nerve. Arch Phys Med Rehabil. 1966; 47: 787-792.
- Bartolic A, Pirtosek Z, Rozman J, Ribaric S. Postural stability of Parkinson's disease patients is improved by decreasing rigidity. Eur J Neurol. 2005; 12: 156-159.
- King LA, Horak FB. Delaying mobility disability in people with Parkinson disease using a sensorimotor agility exercise program. Phys Ther. 2009; 89: 384-393.