Case Report
Is it Feasible to Concomitantly Resect Gastric Gastrointestinal Stromal Tumor during Bariatric Laparoscopic Sleeve Gastrectomy?
Muhammad Al-Zetawi, Hisham Shabani and Samer Al Sawalhi*
Department of Surgery, Al-Essra' Hospital, Amman, Jordan
*Corresponding author: Samer Al Sawalhi, Department of surgery, Al-Essra' Hospital, P O Box 1817-11941) Queen Rania St, Amman, Jordan
Published: 27 Apr, 2017
Cite this article as: Al-Zetawi M, Shabani H, Al Sawalhi S. Is it Feasible to Concomitantly Resect Gastric Gastrointestinal Stromal Tumor during Bariatric Laparoscopic Sleeve Gastrectomy?. Ann Clin Case Rep. 2017; 2: 1346.
Abstract
Laparoscopic Sleeve Gastrectomy (LSG) is one of the bariatric procedures used to ameliorate multiple comorbidities associated with obesity. Moreover, the incidence of Gastrointestinal Stromal Tumors (GISTs) has been found to be more in obese patients. We noticed incidentally a round shape mass, 3 cm x 3 cm in size in the anterior wall of the stomach, closer to the lesser curvature during LSG. The mass was resected successfully laparoscopically with guidance of the endoscope. The frozen section result confirmed the presence of GIST tumor with free margins. GISTs wedge resection during LSG can be done safely depending on the location of the tumor.
Keywords: Laparoscopic sleeve gastrectomy(LSG); Roux-en-Y gastric bypass (RYGB); Gastrointestinal stromal tumors (GISTs); Gastrointestinal (GI); Body Mass Index (BMI)
Introduction
Laparoscopic Sleeve Gastrectomy (LSG) is considered one of the treatment options to ameliorate co-morbidities associated with obesity [1]. However, incidental pathological findings can create a dilemma that may alter the surgical plan. Gastric Gastrointestinal Stromal Tumors (GISTs) are spindle-cell, epitheloid, or, occasionally, pleomorphic mesenchymal tumors that typically arise in the gastrointestinal tract from interstitial pacemaker cells of Cajal and account for <3% of all gastrointestinal neoplasms [2]. GISTs are caused by mutations in the c-kit tyrosine kinase protein or in platelet-derived growth factor receptor alpha (PDGFRα) [3]. Special attention must be paid by surgeons performing LSG, as gastric GISTs are most commonly located in the upper half of the stomach, in accordance with the distribution of interstitial cells of Cajal [4]. Laparoscopic resection of gastric GISTs has been proven to be both safe and effective [5].
Case Presentation
A 34-year-old man who had failed dietary modification for obesity visited our clinic for weight
reduction. He complained of low back pain present for the previous 6 months and was advised to
lose weight. Upon examination, his calculated body mass index was 40.4 (kg/m2), (weight, 110 kg;
height, 165 cm). We discussed options for weight reduction and decided to perform laparoscopic
sleeve gastrectomy (LSG). His values for preoperative tests were all within normal limits except for
the presence of fatty liver detected by abdominal ultrasound. A preoperative barium swallow did not
demonstrateany gastric mucosal abnormalities. The patient did not complain of symptoms related
to gastroesophageal reflux disease. Informed consent was obtained, and the patient was admitted for
LSG. Per usual, five trocars were inserted (two 5 mm trocars into the epigastrium and left anterior
axillary line, two 12 mm trocars into the right and left midclavicular line, and one 10 mm trocar
between the xiphisternum and the umbilicus). After devascularization of the greater curvature of
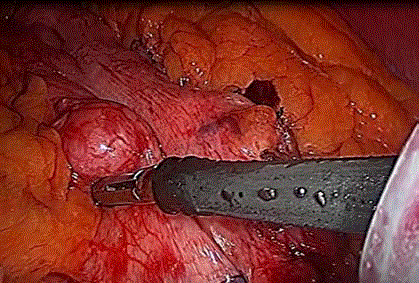
the stomach and insertion of a size 40 bougie, we noticed a lesion in the anterior wall of the stomach,
close to the lesser curvature and in the mid-gastric body. The lesion was rounded in shape, 3 cm x 3
cm in size, covered with serosa, and not ulcerated (Figure 1).
Intraoperative Upper Gastrointestinal (GI) endoscopy was performed to exclude the presence
of any mucosal involvement, to assess the adequacy of the resected margins, and to avoid luminal
narrowing. The endoscopy showed that the mass was located submucosally without ulceration. After
discussion with the patient’s family, the decision was made to laparoscopically resect the mass using
an endoscopic gastrointestinal anastomotic (endo GIA 60, Tri-Stapler Technology–Medtronic,USA)
stapler, which was performed successfully underboth laparoscopic and endoscopic vision. The mass was pulled anteriorly, and a 60 mm endo GIA was used for wedge
resection. The specimen was retrieved in an (endocatch Gold 10 mm
Covidien, USA) without spillage. We waited for frozen sections,
which confirmed the diagnosis of GIST (Figure 2). We continued
with a sleeve resection of the greater curvature of the stomach using
the (endo GIA 60, Tri-Stapler Technology –Medtronic, USA) after
calibration with a 40Fr bougie. Intraoperatively, we tested the integrity
of the sleeve by injecting methylene blue dye (which was negative for
leakage), and an intra-abdominal drain was inserted. The resected
GIST staple line was not included in the resected specimen as it was
anteriorly located near the lesser curvature. The patient underwent a
Gastrografin upper GI study on the third post-operative day torule
out any leak or stenosis. The patient was discharged on the same day
after drain removal and had an uneventful recovery.
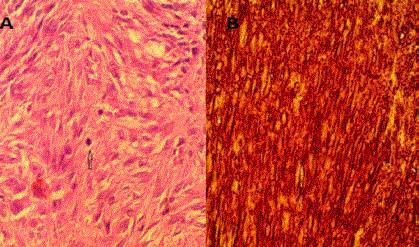
Histopathologic evaluation of the resected specimen showed a
gastric mass (GIST), 2 cm in size with free margins (with a minimum
margin of 1 cm). The mass was found to be a low-grade GIST that
stained positive for CD117 and CD34 but was negative for actin. The
mitotic count was 4/10 cells in a high-power field (Figure 2), therefore
based on the tumor size and mitotic figure, it considered low risk for
recurrence and metastasis.
The patient returned for follow-up at 1and 6 months. Upper
gastrointestinal endoscopy and a computed tomography scan were
negative for recurrence or metastasis. There was no local or port
site recurrence. The patient did not receive post-operative imatinib
mesylate due to a low risk of recurrence according to the National
Comprehensive Cancer Network (NCCN) guidelines version 2, 2017
[6].
Figure 1
Figure 1
A lesion was rounded in shape, 3 cm x 3 cm in size, covered with
serosa, and not ulcerated in the anterior wall of the stomach, close to the
lesser curvature and in the mid-gastric body.
Figure 2
Figure 2
(A) Histopathologic evaluation of the resected specimen showed
a cellular tumor composed of spindle shaped cells arranged in bundles
and fascicles with many ovals to elongated nuclei. No necrosis is seen (B)
Immunostaining positive for CD117 and CD34. The mitotic count was 4/10
cells in a high-power field.
Discussion
The incidence of GIST has been proposed to be higher in patients
with obesity (0.6-0.8%) compared to the generalpopulation (0.0006-
0.0015%) [7,8]. GIST's are difficult to diagnose preoperatively despite
routine upper GI barium tests for all of our patients. Moreover, it
has been shown that routine upper GI endoscopy prior to surgery is
not reliable for identifying GISTs [9]. Surgery is the first-line therapy
for patients with primary resectable GISTs. The objective of surgical
resection of a primary GIST is complete gross resection without
rupturing the tumor pseudo capsule [3]. Novitsky “et al.” [10] demonstrated that a laparoscopic approach appears to offer excellent
therapeutic outcomes, with a long-term disease-free survival for
patients with gastric GIST tumors of various sizes (1.0 cm to 8.5 cm) up to 92%. Additionally Tabrizian “et al.” [11] Showed that patients
undergoing laparoscopic wedge GIST resection have an overall
survival rate of 89% and an overall disease-free survival rate of 77%
for gastric GISTs, with a recurrence rate of 6%.
Recent reports from the National Comprehensive Cancer
Network (NCCN) recommended that a laparascopic approach may
be considered for selected GISTs in favorable anatomic location
(greater curvature or anterior wall of stomach) by surgeons with
appropriate laparascopic experience [6]. It has been recommended
that before resection, both the anterior and posterior surfaces of the
stomach be examined with care. Because some masses are embedded
in fat, a tumor in the lesser curvature might not be recognized. If
a GIST is found during surgery, the entire abdominal cavity and
particularly the liver should also be inspected for metastasis and
secondary tumors [7].
The presence of a tumor on the lesser curvature poses a challenge
and may necessitate a change in the surgical plan from LSG to RYGB
or even termination of the procedure altogether [7] in favor of an
appropriate method of tumor excision. In the case detailed here, we
noticed the gastric mass after inserting the bougie, and it was therefore
more practical to explore the stomach surface after its insertion.
The tumor size and mitotic rate are used as a guide to predict the
malignant potential of GISTs, although it is notoriously difficult to
predict the biological behavior of GISTs based on pathologic features
a lone [6].
In the case of the patient described here, the tumor was considered
low risk. Therefore, resection with a safe margin was curative, and
imatinib was not indicated according to the NCCN guidelines [6].
We decided to perform abdominal computed tomography scanning
6 months after surgery and again yearly.
According to the European Society for Medical Oncology
consensus recommendations, GISTs should be considered for
molecular analysis for KIT or PDGFR-α mutations [12]. Experts
recommend genotyping for all high risk patients, primary imatinib
resistance and metastatic GISTs [13]. Low-risk tumors and those fully
resected do not require this type of testing.
The limitation of our case is that we were unable to diagnose
the tumor prior to surgery, as we do not routinely perform upper
endoscopy before bariatric surgery except for symptomatic patients
and patients undergoing gastric bypass. Still, the significance
of routine pre-operative screening by upper GIT endoscopy in asymptomatic patients remains debatable [8].
Conclusion
GIST wedge resection during laparoscopic sleeve gastrectomy can be performed safely, depending on the location of the tumor. The surgeon should explore the precise location of the lesion to assess the feasibility of sleeve gastrectomy without causing narrowing or tortuosity in the stapler line. Concomitant sleeve gastrectomy and tumor resection is feasible with a laparoscopic approach, giving priority to adequate tumor excision.
References
- Gender Influence on Long-Term Weight Loss and Comorbidities after Laparoscopic Sleeve Gastrectomy and Roux-en-Y Gastric Bypass: a Prospective Study With a 5-Year Follow-up. Obesity Surgery. 2016;26(2):276–81.
- Sasaki A, Koeda K, Obuchi T, Nakajima J, Nishizuka S, Terashima M, et al. Tailored laparoscopic resection for suspected gastric gastrointestinal stromal tumors. Surgery. 2010;147(4):516-20.
- Sawalhi S, Al-Harbi K, Raghib Z, Abdelrahman AI, Al-Hujaily A. Behavior of advanced gastrointestinal stromal tumor in a patient with von-Recklinghausen disease: Case report. World J Clin Oncol. 2013;10(4):70-4.
- Yun HY, Sung R, Kim YC, Choi W, Kim HS, Kim H, et al. Regional Distribution of Interstitial Cells of Cajal (ICC) in Human Stomach. Korean J Physiol Pharmacol. 2010;14(5):317-24.
- Julio Sokolich, Christos Galanopoulos, Ernest Dunn, Jeffrey D. Linder, et al. Expanding the Indications for Laparoscopic Gastric Resection for Gastrointestinal Stromal Tumors. JSLS. 2009;13(2):165–9.
- NCCN guidelines. Gastrointestinal stromal tumors (GIST). version 2, 2017.
- Yuval JB, Khalaileh A, Abu-Gazala M, Shachar Y, Keidar A, Mintz Y, et al. The true incidence of gastric GIST-a study based on morbidly obese patients undergoing sleeve gastrectomy. Obes Surg. 2014;24(12):2134-7.
- Sanchez BR, Morton JM, Curet MJ, Alami RS, Safadi BY. Incidental finding of gastrointestinal stromal tumors (GISTs) during laparoscopic gastric bypass. Obes Surg. 2005;15(10):1384–8.
- De Palma GD, Forestieri P. Role of endoscopy in the bariatric surgery of patients. World J Gastroenterol. 2014;20(24):7777-84.
- Novitsky YW, Kercher KW, Sing RF, Heniford BT. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg. 2006;243(6):738-45.
- Tabrizian P, Nguyen SQ, Divino CM. Laparoscopic management and longterm outcomes of gastrointestinal stromal tumors. J Am Coll Surg. 2009;208(1):80-6.
- ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(7): vii49–55.
- Debiec-Rychter M, Dumez H, Judson I, Wasag B, Verweij J, Brown M, et al. Use of c-KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2004;40(5):689–95.