Case Report
A Case Report on Recurrent Oral Ulcers Associated with Cyclic Neutropenia
Yao Liu#, Jie Fu#, Jie Zhang, Ying Wang and Xiaobing Guan*
Department of Oral Medicine, Capital Medical University, Beijing, China
#These authors contributed equally to this work
*Corresponding author: Xiaobing Guan, Department of Oral Medicine, Beijing Stomatological Hospital, Capital Medical University, No.4 Tiantanxili, Doncheng District, Beijing, China
Published: 23 Mar, 2017
Cite this article as: Liu Y, Fu J, Zhang J, Wang Y, Guan
X. A Case Report on Recurrent
Oral Ulcers Associated with Cyclic
Neutropenia. Ann Clin Case Rep. 2017;
2: 1314.
Abstract
A 12-year-old boy had recurrent oral ulcer and gingival necrosis accompanied with fever, pharyngalgia and painful neck lymph nodes since six months of age. Laboratory examinations revealed an oscillation of peripheral blood neutrophils from normal to severely low levels, with 21-day periodicity. A mutation in exon 4 of neutrophil elastase 2 (ELA2) was identified. The symptoms and lab examination led to the diagnosis of cyclic neutropenia. After the patient received recombinant human granulocyte colony-stimulating factor (rhG-CSF) treatment around the early neutropenic phase, the symptoms abated and peripheral blood neutrophil counts returned to normal levels. This case demonstrates the need for oral medicine clinicians to perform testing for cyclic neutropenia when patients are afflicted with recurrent oral ulcers.
Keywords: Oral ulcer; Cyclic neutropenia; Neutrophil elastase 2
Introduction
Cyclic Neutropenia (CyN) is a rare blood disorder characterized by regular oscillations in the value of peripheral blood neutrophils, generally with 21-day periodicity [1]. CyN was first reported in 1910, and usually presents early in childhood [2]. An estimated incidence of CyN was one to two per million [3]. Absolute neutrophil count is nadir, and always below 0.2*109/L [4]. During phases of neutropenia, patients frequently suffer from fever, necrotic stomatitis, pharyngalgia, lymphadenopathy and more serious infections. With increasing neutrophil count, the infections disappear [5,6]. CyN is responsive to granulocyte colony-stimulating factor (G-CSF), whereby G-CSF administration leads to shortened nadir duration, increased mean neutrophil count and alleviated clinical symptoms, but does not prevent recurrence [4]. Recently the locus for autosomal dominant CyN was mapped to chromosome 19p13.3, and CyN is now attributable to mutations found in the gene encoding neutrophil elastase (the ELA2 gene) [5]. Eighty percent of CyN patients are reported as having an ELA2 mutation. Several studies reported that CyN ELA2 mutations tend to be located in intron 4 and exons 2, 3 and 4 [4,5]. Here, we report a case of CyN in a 12-year-old boy as determined through clinical and lab examinations and by demonstration of a mutation in exon 4 of the ELA2 gene. There is an association of oral ulcers with cyclic neutropenia and this should be taken into consideration by examining physicians.
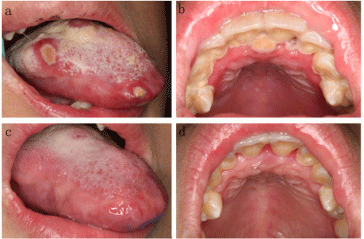
Figure 1
Figure 1
Oral symptoms (12-year-old, male). (a) Several major ulcers on
tongue mucosa with a yellow pseudomembrane; (b) Generalized gingival
necrosis with gingival congestion; (c-d) The ulcers on tongue mucosa and
gingival necrosis abated, when the patient’s neutrophil counts returned to
normal.
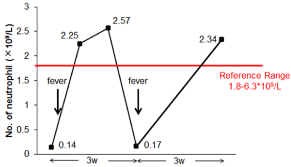
Figure 2
Figure 2
Oscillation of peripheral counts. Neutrophil count oscillates with a
periodicity of 21 days in a 12-year-old male.
Case Presentation
A 12-year-old Chinese boy experienced year-long signs of oral ulcers and fever, with a cycle
time of 3 weeks. The patient’s medical history indicated that he had suffered from oral ulcers,
gingival necrosis and fever accompanied with pharyngalgia and painful neck lymph nodes since
he was six months of age. Oral examination revealed oral ulcers and gingival necrosis. Four
necrotic ulcers were found on the tongue mucosa. The ulcers were covered by a yellow and thick
pseudomembrane (Figure 1A). Gingival margin and papilla were necrosed, common in upper and
mandibular anterior teeth. The necroses were covered by yellow pseudomembrane, with easily
hemorrhage and special septic halitosis. The gingival around necroses were congestion (Figure
1B). Poor oral hygiene was also observed, along with accumulation of bacterial plaque and food
debris. In addition, the patient’s neck lymph nodes were pain and enlargement, pharynx mucosa
was congestion. By this time, laboratory examination revealed a neutrophil count of 0.14*109/L.
His neck lymph nodes ultrasonography demonstrated bilateral neck lymphadenopathy. Around 21
days later, the signs, including oral ulcers, gingival necrosis, lymphadenopathy and congestion of
pharynx mucosa, were dissipated (Figure 1C and D), At this moment, his neutrophil count returned to 2.25*109/L. Several blood routine tests have been carried out on the
patient, showed severely low peripheral blood neutrophils count with
21-day periodicity (Figure 2). The patient was diagnosed with cyclic
neutropenia based on his oral manifestations, cyclical decreased
neutrophil count with 21-day periodicity. However, CyN need to
differential diagnose with other diseases, especially agranulocytosis
and periodic fever aphthous-stomatitis pharyngitis cervical-adenitis
syndrome (PFADA syndrome). Agranulocytosis clinic was mainly
included necrotic ulcers, gingival necrosis. While neutrophil count
was below 0.5*109/L, but without periodicity. PFADA syndrome was
characterized by periodic fever, aphthous stomatitis, pharyngitis
and adenitis, those were similar with CyN. However, the patient’s
neutrophil count was not decreased.
Blood was collected from the patient and both parents. All
procedures and consents were approved by human subjects of
committees of Beijing Stamotological Hospital, and they signed
informed consent forms. DNA was isolated from the peripheral blood
using the DNeasy Blood Tissue Kit (Qiagen, Germany) according to
the manufacturer’s recommended procedures. PCR amplification
of the affected sequence in the ELA2 gene was performed with
two primers 5’-TGGCAGGCACTCAGCA-3’ (F)/5’-GGGGTCG
TAGCCGTTTTC-3’ (R) and 5’-GCACTCAAGCCACATCC-3’
(F)/5’-TCAACACCCAATCACACAG-3’ (R). DNA regions found
between these two primers contain 13 mutation locations (Table
1). The resulting amplicons were sequenced using a sequencing kit
(Applied Biosystems, Foster City, CA) and ABI PRISM®3730XL Genetic analyzer. The sequence examination identified the patient’s
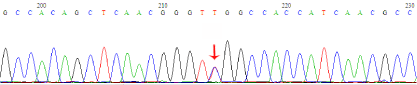
ELA2 gene had a mutation in exon 4, with a single nucleotide change
(4495C>T), however, as this mutation was not found in either of his
parents it was considered a de novo mutation (Figure 3 and Table
1). The patient was advised to receive subcutaneous injections of
recombinant human granulocyte colony-stimulating factor (rhGCSF)
(150 μg) at the early neutropenic phase, until neutrophil counts
returned to normal. The patient was also prescribed pidotimod (800
mg per day) along with tinidazole (1g per day) for gingival necrosis. In
addition, the patient was advised to use 1% povidone iodine solution
as a mouth wash three times per day while he presented oral ulcers.
Two year following treatments, the patient’s symptoms relieved and
his peripheral blood neutrophils counts returned to normal levels.
Figure 3
Figure 3
DNA sequence result of CyN patient. Sequence analysis identified
1 mutation in ELA2 gene. 4495C>T mutation (arrow showed).
Discussion
CyN was diagnosis by regular oscillations of peripheral blood
neutrophils from normal to severaly low levers, generally with 21-
days periodicity [4]. The diagnosis depends on serial measurements
of absolute nertrophil counts over a period of several weeks [5,6]'. This
patient was diagnosed with CyN as he was determined to have severely
low peripheral blood neutrophils count with 21-day periodicity
associated with fever, oral ulcers, gingival necrosis, pharyngalgia and
neck lymphadenopathy, followed by a recovery phase during where
his peripheral neutrophil levels returned to normal. CyN occurs both
as a childhood-onset and adult-onset disease by taking account of
age. While Cyn generally first presents in childhood, for nearly 25%
of patients’, the first symptoms present after age 20 [1]. Affected
individuals typically experience recurrent aphthous stomatitis, fever,
malaise, pharyngitis, sinusitis, or more serious infections (such as
colitis with gram negative sepsis) [4,7]. The mechanisms driving
CyN is still unknown. A possible mechanism for childhood-onset
CyN is associated with an underlying disturbance in the granulocytemacrophage
colony-stimulating factor (GM-CSF) responsive growth of myeloid progenitors committed to neutrophilic differentiation
[8]. Interestingly, rhGM-CSF treatment is not effective at increasing
neutrophil counts in all CyN patients suggesting that a lack of GMCSF
in patients with CyN does not entirely explain the mechanisms
of CyN development [9]. Recently, several studies reported positional
cloning studies that mapped the locus for autosomal dominant
CyN to chromosome 19p13.3, the locus for several serine proteases,
and this disease is now attributable to multiple mutations found
in the gene encoding ELA2 [5]. ELA2 gene is activated primarly
at the promyelocytic stage during neutrophil development [4,10].
Mutations found in this gene result in accelerated apoptosis in
differentiating myeloid cells [4,11]. Additionally, Ela2-/- mice do not
have deficient neutrophil counts, but do have impaired intracellular
killing of bacteria by neutrophils and are susceptible to death
following intraperitoneal infection with Gram-negative bacteria [12].
ELA2 gene mutations have been found in 80-100% of CyN cases
[4] Most of splice-site mutations cluster in the splice-donor region
of intron 4, and others were located in exons 2, 3, 4 and 5 [3]. The
mutation type were missense, splicing defect and in-frame deletions,
such as 1847C>A in exon2, 4495C>T and 4675-4715 del in exon4 and
IVS4+1 G>A in intron4. At the protein level, these mutations resulted
in amino acid substitutions, such as F14L, S17F and S97L [3,4]. In
our study, sequencing of the patient’s ELA2 gene demonstrated a
mutation in exon 4 (4495C>T). This missense mutation 4495C>T is
responsible for the S97L substitution. Genotype-phenotype analysis
strongly suggests that ELA2 mutations are correlated with more severe
presentation of neutropenia [3]. Colony stimulating factor 3 receptor
is another gene associated with congenital neutropenia and CyN
[13]. These studies elucidate several genetic disruption and molecular
mechanisms leading to CyN, thus providing important insights into
potential mechanisms of CyN development. Those new insights could
improve diagnosis and treatment for CyN patients. Adult-onset CyN
can be treated with corticosteroids or immunosuppressants, whereas
childhood-onset CyN is not responsive to these treatments. Recently,
a positive outcome reported in patients treated through systemic
administration of G-CSF suggests that G-CSF is a potentially effective
treatment of childhood-onset CyN patients [14]. RhG-CSF treatment
has been shown to result in substantially increased average neutrophil
counts in patients with childhood-onset CyN [15]. Contrasting
effects of G-CSF and GM-CSF treatment on CyN found that G-CSF
increased average neutrophil counts more than 20-fold, and GM-CSF
increased neutrophil counts only modestly. Another study showed
that G-CSF treatment significantly increased the patient’s average
neutrophil counts while at the same time amplifying neutrophil
cycling and reducing the duration of neutropenic nadir and the
frequency of skin and pulmonary infections [16]. Recently, researchers
demonstrated that intramuscular administration of G-CSF-lentivirus
to a normal dog and a gray collie elevated the neutrophil levels for
more than 5 months, significantly increased neutrophil counts over
the pretreatment level, with no adverse effects. G-CSF delivery by
gene therapy using lentiviral vectors could also be a mode of delivery
for a long-term treatment of patients with CyN [17]. However, more
studies need to be performed to determine its efficacy and safety as a
clinical treatment.
In summary, this report presents a rare case of childhoodonset
CyN. A thorough review of the patient’s medical history
and monitoring of neutrophil counts were essential to make this
diagnosis. Treatment with rhG-CSF resulted in a significant increase
in neutrophil counts and reduction in associated symptoms. Since
oral ulcer occurs in all CyN patients, dentist should be aware of the association between recurrent oral ulcer and cyclic neutropenia in order to make give early diagnosis and treatment.
References
- Wright DG, Dale DC, Fauci AS, Wolff SM. Human cyclic neutropenia: clinical review and long-term follow-up of patients. Medicine (Baltimore). 1981;60(1):1-13.
- Leale M. Recurrent furunculosis in an infant showing an unusual blood picture. JAMA. 1910;liv(23):1854-5.
- Bellanne-Chantelot C, Clauin S, Leblanc T, Cassinat B, Rodrigues-Lima F, Beaufils S, et al. Mutations in the ELA2 gene correlate with more severe expression of neutropenia: a study of 81 patients from the French Neutropenia Register. Blood. 2004;103(11):4119-25.
- Newburger PE, Pindyck TN, Zhu Z, Bolyard AA, Aprikyan AA, Dale DC, et al. Cyclic neutropenia and severe congenital neutropenia in patients with a shared ELANE mutation and paternal haplotype: evidence for phenotype determination by modifying genes. Pediatr Blood Cancer. 2010;55(2):314-7.
- Dale DC, Person RE, Bolyard AA, Aprikyan AG, Bos C, Bonilla MA, et al. Mutations in the gene encoding neutrophil elastase in congenital and cyclic neutropenia. Blood. 2000;96(7):2317-22.
- Inoue T, Tani K, Tajiri M, Ishida Y, Seguchi M, Tanaka H, et al. A case report of familial cyclic neutropenia. Tohoku J Exp Med. 1992;167(2):107- 13.
- Block MS, Brindis M, Block CA, Berron JM. Full-Arch Rehabilitation of a Patient With Cyclic Neutropenia. J Oral Maxillofac Surg. 2015;73(9):1734.
- Wright DG, LaRussa VF, Salvado AJ, Knight RD. Abnormal responses of myeloid progenitor cells to granulocyte-macrophage colony-stimulating factor in human cyclic neutropenia. J Clin Invest. 1989;83(4):1414-8.
- Hammond WP, Chatta GS, Andrews RG, Dale DC. Abnormal responsiveness of granulocyte-committed progenitor cells in cyclic neutropenia. Blood. 1992;79(10):2536-9.
- Papadaki HA, Eliopoulos GD. The role of apoptosis in the pathophysiology of chronic neutropenias associated with bone marrow failure. Cell Cycle. 2003;2(5):447-51.
- Köllner I, Sodeik B, Schreek S, Heyn H, von Neuhoff N, Germeshausen M, et al. Mutations in neutrophil elastase causing congenital neutropenia lead to cytoplasmic protein accumulation and induction of the unfolded protein response. Blood. 2006;108(2):493-500.
- Belaaouaj A, McCarthy R, Baumann M, Gao Z, Ley TJ, Abraham SN, et al. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat Med. 1998;4(5):615-8.
- Klimiankou M, Mellor-Heineke S, Klimenkova O, Reinel E, Uenalan M, Kandabarau S, et al. Two cases of cyclic neutropenia with acquired CSF3R mutations, with 1 developing AML. Blood. 2016;127(21):2638-41.
- Chen LL, Toyoguchi M, Shimakawa M, Hori S. Chronic refractory uveitis in a patient with childhood-onset cyclic neutropenia. Case Rep Ophthalmol. 2011;2(2):155-9.
- Wright DG, Kenney RF, Oette DH, LaRussa VF, Boxer LA, Malech HL. Contrasting effects of recombinant human granulocyte-macrophage colony-stimulating factor (CSF) and granulocyte CSF treatment on the cycling of blood elements in childhood-onset cyclic neutropenia. Blood. 1994;84:1257-67.
- Horwitz MS, Duan Z, Korkmaz B, Lee HH, Mealiffe ME, Salipante SJ. Neutrophil elastase in cyclic and severe congenital neutropenia. Blood. 2007;109(5):1817-24.
- Yanay O, Dale DC, Osborne WR. Repeated lentivirus-mediated granulocyte colony-stimulating factor administration to treat canine cyclic neutropenia. Hum Gene Ther. 2012;23(11):1136-43.