Case Report
Metastatic Melanoma Responding to Combination Chemotherapy and Immunotherapy after Progression on Ipilimumab and Nivolumab Immunotherapy
Elen Vettus1, David Draper2 and Michael A Davies3*
1Department of Chemotherapy, Oncology and Haematology Clinic, North Estonia Medical Center, Estonia
2Department of Medical Oncology, Tri Health Cancer Institute, USA
3Department of Melanoma Medical Oncology, The University of Texas MD Anderson Cancer Center, USA
*Corresponding author: Michael A. Davies, Department of Melanoma Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, Texas, 77030 USA
Published: 30 Jan, 2017
Cite this article as: Vettus E, Draper D, Davies MA.
Metastatic Melanoma Responding
to Combination Chemotherapy and
Immunotherapy after Progression
on Ipilimumab and Nivolumab
Immunotherapy. Ann Clin Case Rep.
2017; 2: 1251.
Abstract
We report the case of a patient with stage IV M1c melanoma that developed symptomatic disease progression after treatment with combination immunotherapy (ipilimumab with nivolumab). The patient was subsequently treated with nab-paclitaxel chemotherapy and pembrolizumab and had a rapid and dramatic clinical response. The case illustrates that clinical responses are possible after progression on combination immunotherapy.
Keywords: Metastatic melanoma; Nab-paclitaxel; Pembrolizumab; Ipilimumab; Nivolumab; Resistance
Introduction
The World Health Organization (WHO) estimates that the annual world-wide incidence of
cutaneous melanoma is 132,000 cases per year1. Outcomes for patients with stage IV melanoma
have historically been poor. In the 7th edition of the AJCC guidelines, the median survival for stage
IV was ~8 months and the five year survival rate was <10% [1]. However, treatments and outcomes for these patients are rapidly changing.
The first agent approved by the United States Food and Drug Administration (FDA) for the
treatment with stage IV melanoma was the cytotoxic chemotherapy dacarbazine (DTIC) [2].
Dacarbazine achieves clinical responses in 5-10% of patients, most of which are short-lived. The
immunotherapy high dose interleukin-2 (HD IL-2) was approved for stage IV melanoma in
1998. While response rates remained low (~15%), patients that achieve complete responses (CR)
frequently achieve long-term surival (i.e. > 10 years) [3]. Unfortunately, the CR rate for HD IL-2
therapy is only ~6%, and the treatment-related mortality is 1-2%. From 1998 to 2011 there were
no new agents approved for patients with stage IV melanoma. However, from 2011 to 2016 five
targeted therapy and 4 immunotherapy regimens have been approved by the FDA [4-14]. Targeted
therapies are currently approved only for patients with BRAFV600 mutations, which are detected in
40-50% of cutaneous melanomas. In contrast, immunotherapies may induce response regardless
of genetic abnormalities. The approved immunotherapies include the anti-CTLA-4 antibody
ipilimumab (FDA approval, 2011); the anti-PD-1 antibodies nivolumab (2014) and pembrolizumab
(2014); and the combination regimen of ipiliumab and nivolumab (2015). In the recent Checkmate
067 study, treatment with ipilimumab monotherapy, nivolumab monotherapy, and ipilimumab +
nivolumab combination therapy achieved objective responses in 19%, 44%, and 58% of treamentnaive
metastatic melanoma patients [9]. Additional reports have demonstrated that ipilimumab
+ nivolumab can achieve very rapid clinical benefit in patients, and can achieve durable disease
control even in patients with high serum lactate dehydrogenase (LDH) levels, a known negative
prognostic factor in stage IV melanoma [15,16].
These results have transformed first-line treatment of patients with metastatic melanoma.
However, there is very little known about the efficacy of systemic therapies in patients who have
progressed on immunotherapy, particularly after combination immunotherapy with ipilimumab
and nivolumab. Here we describe the case of a metastatic melanoma patient with a wild-type BRAF
gene that developed symptomatic progression after treatment with ipilimumab + nivolumab.
Case Presentation
The patient presented at age 11 with a pigmented lesion of the left
ear. A shave biopsy revealed melanoma in situ. A subsequent wide local
excision revealed no residual disease. At age 27 the patient presented
to an emergency room with complaints of fatigue, night sweats, and
weight loss of 1 month duration. Diagnostic testing identified elevated
liver enzymes and MRI of the abdomen demonstrated innumerable
masses in the liver, with 50-75% replacement of normal liver tissue.
CT scans revealed subcarinal adenopathy, liver metastases, an
enlarged lymph node in the mesenteric root, and in the left pelvic
sidewall, and rectal wall thickening near the anorectal verge with
associated perirectal lymphadenopathy. MRI of the brain showed
no abnormalities. A biopsy of a liver mass confirmed metastatic
melanoma. Molecular testing on the biopsy detected no mutations
in the BRAF, NRAS, or c-KIT genes, and immunohistochemistry
failed to identify expression of PD-L1 protein by the tumor cells.
Colonoscopy revealed a 5 cm by 8 cm discolored rectal mass with
an ulcerated center; rectal brushings confirmed the diagnosis of
metastatic melanoma.
The patient started treatment with the combination
immunotherapy regimen ipilimumab (3 mg/kg) and nivolumab (1
mg/kg) six weeks after initial presentation. At the start of treatment,
the serum LDH level was approximately 6 times greater than the
upper limit of normal (ULN). The patient’s symptoms improved
significantly after the first cycle of treatment and the second cycle
was given as scheduled three weeks later. The patient subsequently
developed symptoms of mild colitis, which resolved with oral
budesonide therapy. Standard blood testing demonstrated normal
serum AST, ALT, and LDH levels prior to the third cycle. After the
fourth cycle, the patient developed grade 2 hepatitis with elevated
transaminases, along with fevers and night sweats. The patient was
started on oral prednisone therapy for presumed autoimmune
hepatitis, with rapid resolution of symptoms and transaminitis.
Restaging studies demonstrated progressive disease. The patient
was weaned off steroids, and then started single-agent nivolumab (3 mg/kg every 2 weeks). After 3 cycles of maintenance nivolumab,
the patient noted increasing fatigue, night sweats, and abdominal
distention. Serum LDH was ~18x the ULN, the serum AST ~2x the
ULN, and the patient was anemic (serum hemoglobin of 7.9 g/dl).
CT demonstrated marked increase in the size of hepatic and rectal
metastases, as well as worsening lymphadenopathy in the axilla,
abdomen, and inguinal regions (Figure 1). The patient was screened
for multiple clinical trials, but did not meet inclusion criteria. The
decision was made to treat the patient with nab-paclitaxel (260 mg/
m2) and pembrolizumab (2 mg/kg) given every three weeks. The
first treatment was administered one month after the last dose of
nivolumab, and the patient reported symptomatic improvement after
just a few days. Three weeks later laboratory testing demonstrated
normalization of transaminases and an 85% reduction in LDH. CT
scans performed 3 weeks later showed ~40% reduction in size of the
target liver metastases along with improvement of the other masses.
The patient has now completed 11 cycles (8 months) of nabpaclitaxel
with pembrolizumab treatments. The only side effect the
patient has experienced is grade 1 neuropathy. Testing after cycle 10
demonstrated normal liver enzymes, near-normal serum LDH, and
continued objective response by RECIST criteria (Figure 1).
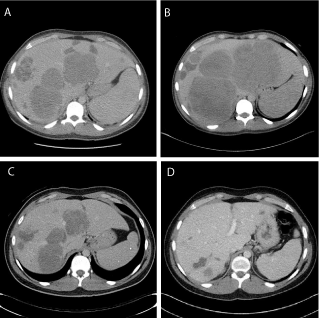
Figure 1
Figure 1
Radiographic response to nab-paclitaxel with pembrolizumab.
Representative images of liver metastases (A) after 4th treatment with
ipilimumab with nivolumab, (B) after 3rd treatment with nivolumab, (C) after
4th treatment with nab-paclitaxel and pembrolizumab, and (D) after 10th
treatment with nab-paclitaxel and pembrolizumab.
Discussion
The care of patients with metastatic melanoma has been
revolutionized by the development of increasingly effective therapies.
The recent approval of ipilimumab and nivolumab has provided a
powerful new therapeutic option for stage IV melanoma patients.
While this therapy achieves clinical responses in 55-60% of patients,
there is a clear unmet need to identify effective treatments for
patients that are resistant to this combination. Current National
Comprehensive Cancer Network (NCCN) Guidelines for the
treatment of patients with metastatic melanoma recommend the
use of a different class of therapy in the second-line setting [17]. For
patients with a BRAFV600 mutation that progress on ipilimumab and
nivolumab, there are multiple effective targeted therapy options.
However, there are currently no targeted therapies that are approved
for patients without a BRAFV600 mutation. While clinical trials are a
good option, often patients are not able to participate in such trials
due to a variety of factors.
Treatment with cytotoxic chemotherapy is a potential secondline
treatment option. However, many oncologists are reluctant
to use chemotherapy in metastatic melanoma patients due to the
low activity of dacarbazine (DTIC), which to date remains the only
cytotoxic agent FDA approved for stage IV melanoma. Alternative
NCCN-recommended options include single-agent paclitaxel, the
combination of paclitaxel with carboplatin, and nab-paclitaxel. A
recently reported phase III trial in 529 stage IV melanoma patients
with serum LDH less than 2-times the ULN demonstrated that nabpaclitaxel
achieved clinical responses in 15% of patients and resulted
in a significant improvement in progression-free survival (PFS)
(median 4.8 versus 2.5 months, hazard ratio (HR) 0.792, p=0.044)
compared to DTIC [18]. Notably, nab-paclitaxel is prepared as a
solvent-free formulation which does not require steroids, which are
generally required for paclitaxel. This trial included very few patients
with previous immunotherapy treatment (6%). The patient in this
case report achieved a rapid and dramatic clinical response with
combined treatment using nab-paclitaxel and pembrolizumab after
progression on ipilimumab and nivolumab. Although the kinetics
of the observed clinical response are consistent with the activity of a cytotoxic agent in this patient, we cannot exclude the possibility
that pembrolizumab contributed to this response. However, there
is no published data demonstrating a difference in activity for
pembrolizumab versus nivolumab, or that pembrolizumab has benefit
after progression on nivolumab. There is also no data demonstrating
benefit for combining cytotoxic chemotherapy with immunotherapy.
It is also possible that the previous exposure to immunotherapy may
have contributed to the response, as the potential for prolonged
effects of immunotherapy are supported by the observation of
delayed autoimmune toxicity from these agents even several months
after they are discontinued, particularly with ipilimumab [19].
Despite this possibility, in the KEYNOTE-002 trial for metastatic
melanoma patients that had progressed on ipilimumab treatment, the
response rate for patients treated with a variety of chemotherapies
(DTIC, temozolomide, paclitaxel, carboplatin, or paclitaxel with
carboplatin) was only 4% [20]. The activity of nab-paclitaxel has
not been evaluated systematically in metastatic melanoma patients
previously treated with immunotherapy, nor has any chemotherapy
after progression on anti-PD-1 (+/- anti-CTLA-4) treatment. In
summary, this case reports illustrates that cytotoxic chemotherapy
may produce significant clinical benefit with acceptable side effects
in patients that have progressed on combination immunotherapy,
even in the setting of active symptoms and elevated LDH levels.
This report supports the rationale to consider such treatments in
appropriately selected patients. Further, in order to inform clinical
decision-making, we believe that there is need to systematically
evaluate cytotoxic chemotherapies in patients previously treated with
anti-PD-1 (+/- anti-CTLA-4), and potentially to compare the effects
with and without continued immunotherapy. Such trials would be
particularly relevant for patients without a BRAFV600 mutation and/or
that are excluded from immunotherapy trials due to prior toxicities.
Foot Note: 1World Health Organization (WHO), http://www.
who.int/uv/faq/skincancer/en/index1.html, accessed December 1,
2016.
References
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009; 27: 6199-6206.
- Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist. 2011; 16: 5-24.
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-Dose Recombinant Interleukin 2 Therapy for Patients With Metastatic Melanoma: Analysis of 270 Patients Treated Between 1985 and 1993. J Clin Oncol. 1999; 17: 2105.
- Merlino G, Herlyn M, Fisher DE, Bastian BC, Flaherty KT, Davies MA, et al. The state of melanoma: challenges and opportunities. Pigment Cell Melanoma Res. 2016; 29: 404-416.
- Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014; 371: 1877-1888.
- Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. The Lancet. 2015; 386: 444-451.
- Larkin J, Ascierto PA, Dréno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014; 371: 1867-1876.
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010; 363: 711-723.
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015; 373: 23-34.
- Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015; 372: 2006-2017.
- Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011; 364: 2517-2526.
- Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015; 372: 30-39.
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015; 372: 320-330.
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015; 372: 2521-2532.
- Larkin J, Ferrucci PF, Gonzalez R, Thomas L, Maio M, Hill A, et al. Efficacy of nivolumab (NIVO) plus ipilimumab (IPI) combination in patients with advanced melanoma (MEL) and elevated serum lactate dehydrogenase (LDH): a pooled analysis. Pigment Cell & Melanoma Research. 2017
- Chapman PB, D'Angelo SP, Wolchok JD. Rapid eradication of a bulky melanoma mass with one dose of immunotherapy. N Engl J Med. 2015; 372: 2073-2074.
- Coit DG, Thompson JA, Algazi A, Andtbacka R, Bichakjian CK, Carson WE, et al. NCCN Guidelines Insights: Melanoma, Version 3.2016. J Natl Compr Canc Netw. 2016; 14: 945-958.
- Hersh EM, Del Vecchio M, Brown MP, Kefford R, Loquai C, Testori A, et al. A randomized, controlled phase III trial of nab-Paclitaxel versus dacarbazine in chemotherapy-naďve patients with metastatic melanoma. Annals of Oncology. 2015; 26: 2267-2274.
- Di Giacomo AM, Biagioli M, Maio M. The emerging toxicity profiles of anti-CTLA-4 antibodies across clinical indications. Semin Oncol. 2010; 37: 499-507.
- Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumabrefractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015; 16: 908-918.