Case Report
Very Late Recurrence of Hepatocellular Carcinoma after Sequential Liver and Kidney Transplant: Is There an Influence of Immunosuppression?
Renato Ferreira da Silva1, Carolina Antunes Marques2, Fábio Leite Couto Fernandez3, Dalisio de Santi Neto4, Helen C Felicio1, Wiliam Jose Duca5, Ida Maria M Fernandez6, Paulo Cesar Arroyo Jr7 and Rita de Cásisa Martins Alves da Silva1
1Department of Surgery and Liver Transplantation, Hospital de Base/ FUNFARME of the São José do Rio Preto Medicine School / FAMERP, Brazil
2Student of São José do Rio Preto Medicine School/FAMERP, Brazil
3Department of Oncology, Hospital de Base/FUNFARME São José do Rio Preto, Brazil
4Department of Pathology, Hospital de Base/ FUNFARME of the São José do Rio Preto Medicine School/FAMERP, Brazil
5Department of Gastroenterology, Hospital de Base/ FUNFARME São José do Rio Preto, Brazil
6Department of Surgery and Kidney Transplantation, Hospital de Base/ FUNFARME of the São José do Rio Preto
Medicine School / FAMERP, Brazil
7Department of General Surgery, Hospital de Base/ FUNFARME São José do Rio Preto, Brazil
*Corresponding author: Renato F da Silva, Unit of Surgery and Liver Transplantation, Hospital de Base/ FUNFARME of the São José do Rio Preto Medicine School/FAMERP, São Paulo, Brazil
Published: 14 Jan, 2017
Cite this article as: da Silva RF, Marques CA, Fernandez
FLC, de Santi Neto D, Felicio HC,
Duca WJ, et al. Very Late Recurrence
of Hepatocellular Carcinoma
after Sequential Liver and Kidney
Transplant: Is There an Influence of
Immunosuppression?. Ann Clin Case
Rep. 2017; 2: 1236.
Abstract
We report a case of a very late recurrence of hepatocellular carcinoma, which occurred after kidney transplantation. Six years earlier, liver transplantation had been performed due to hepatocellular carcinoma. This is the second case reported in the medical literature where a recurrence occurred after a strong immunosuppression regimen. The influence of immunosuppression in the recurrence of hepatocellular carcinoma will be discussed in this case.
Keywords: Liver; Kidney; Transplantation; Immunosuppression; Hepatocellular; Carcinoma; Recurrence; Mycophenolate sodium; Mycophenolate mofetil; Sirolimus and tacrolimus
Introduction
One of the most highly incident cancers in the world is hepatocellular carcinoma (HCC) [1];
it is the fifth most common cancer in males and the cancer second most related to death [2,3].
Usually, the treatment of HCC for patients begins at an early stage of liver disease and with relatively
preserved liver function, but this must consider tumor size and number. Curative treatment options
include tumor resection, radiofrequency ablation and liver transplantation [4].
The Milan criteria are widely accepted for the indication of liver transplantation, leading to 4-
and 5-yearsurvival rates greater than 85% and 70%, respectively. Although several limitations, such
as organ shortage and tumor recurrence, other extended criteria have shown similar 5-yearsurvival
rates ranging from 71 to 87% [1]. The recurrence ratio (10-60%) is related to factors such as tumor
number (especially a tumor size greater than 5 cm), lymphovascular invasion, multifocal tumors,
alpha-fetoprotein level greater than 200 ng/dl, poor differentiation, preoperative transparietal
biopsy, down-staging of the tumor, and additionally, to the influence of the immunosuppressive
drugs [2,3].
Schreibman et al. [1] reported the first case in the literature of a late HCC recurrence after
transplantation that could be due to immunosuppression. We report here the second case in the
medical literature of a late HCC recurrence after sequential liver and kidney transplantation (Table
1).
Case Presentation
A 48-year-old male patient, with type 2 diabetes mellitus, developed secondary cirrhosis due
to alcohol consumption. He was at Child-Pugh B level when he received a non-invasive diagnosis
of HCC with an abdominal tomographic scan, which discovered four nodules measuring less than 2 cm each, on the right lobe, associated with an Alpha Fetoprotein
(AFP) serum level of 350.2 ng/ml.
While waiting for liver transplantation, he underwent two
chemoembolization sessions. His liver transplant occurred in May
2003; he had been registered on the waiting list for sixteen months.
At that time, in Brazil, patients were submitted to transplantation
surgery according to their waiting time on the transplant list;
the MELD system was introduced in 2006. By histopathological
examination of the explanted liver, we found that he was beyond the
Milan criteria, showing multifocal hepatocellular carcinoma spread
over all liver segments (I to VIII), with tumor diameters of 1.6 to 1.7
cm, trabecular and pseudo-glandular occurrences, Edmonson-Steiner
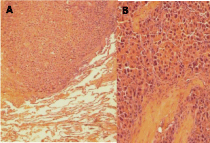
grade III, and also foci of micro- and macro-vascular invasion (Figure
1A and B). The TNM classification was T4N0Mx, as the two lymph
nodes removed from the peripancreatic and portal region were free of
malignancy. After liver transplantation, the alpha-fetoprotein serum
level fell from 350 ng/ml to 4.4 ng/ml.
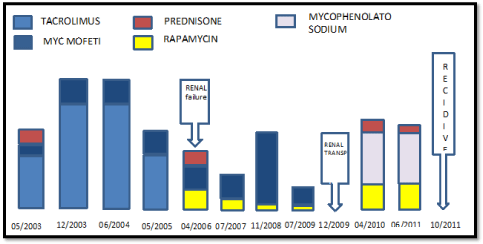
On the day after liver transplantation, immunosuppression was started with prednisone 20 mg per day, tacrolimus 2 mg every
12h and mycophenolate mofetil 500 mg daily. After 21 days of
immunosuppression, prednisone was decreased by 5 mg every three
weeks, until it was stopped at the end of the third month, as required
by our pre-stated protocol. The serum level of tacrolimus ranged
from 2 to 13 ng/mL (mean of 8.37 ng/ml).
In 2006 April, three years after the liver transplantation, due
to severe diabetes complications, tacrolimus had been replaced by
sirolimus, with an initial dose of 2 mg per day, adjusted to 1 mg on
alternate days. The serum sirolimus level ranged from 2.5 to 6.2 ng/
mL (mean of 3.88 ng/mL) throughout the three subsequent years.
Mycophenolate mofetil was maintained throughout the period with
adjusted doses, according to clinical and laboratorial evaluations.
Six years after the liver transplantation (December 2009), the
patient underwent a heterologous kidney transplantation from a
cadaveric donor due to chronic kidney failure, as a consequence of
diabetic nephropathy. The preoperative evaluation, including bone
scintigraphy, thorax and abdominal tomographic scans and serum
alpha-fetoprotein, showed no evidence of recurrence of the HCC.
After the kidney transplant, the immunosuppressive regimen was changed to thymoglobulin (ATG), 4.5 mg/kg divided in three doses
and methylprednisolone 500 mg, followed by prednisone 45 mg/day
(0.5 mg/kg) and sodium mycophenolate 720 mg every 12 h. Sirolimus
was maintained on 1 mg every day. On the eleventh day after the
kidney transplant had occurred, acute rejection of the new organ was
treated with a methylprednisolone pulse, 1 g for three days. Then,
immunosuppression was maintained with prednisone 45 mg/day,
sodium mycophenolate 720 mg every 12h and sirolimus 2 mg/day.
Eighteen months after the renal transplant (June 2011), routine
exams showed an increasing alpha-fetoprotein level from 6.7 ng/mL
to 232.6 ng/ml. Bone scintigraphy showed no evidence of metastasis.
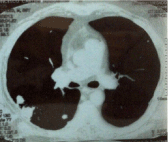
However, a computed tomography chest scan identified nodules
with a diameter of 2.5 cm in the right lung (Figure 3). The patient
was referred for pulmonary lobectomy, which was not performed
due to disseminated tumors on the lungs and pleura. Upon
histopathological examination, metastatic HCC in the lungs was
diagnosed, 8.5 years after liver transplantation, as demonstrated in
Figures 4A and 4B. Thereafter, the patient was treated with sorafenib
400 mg twice daily for 80 days, when the treatment was discontinued
due to the progression of the metastatic liver tumor. Second-line
systemic therapy with gemcitabine and oxaliplatin started, but it was
suspended after the second cycle due to myelotoxicity. The patient
underwent supportive therapy until progression to death, eleven
months after the HCC recurrence.
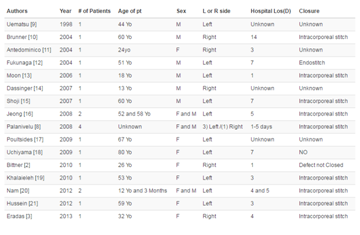
Table 1
Table 1
Demographics and clinical characteristics of the two reported patients with very late HCC recurrence after Liver transplantation.
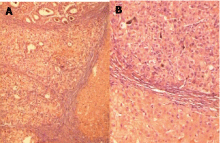
Figure 1
Figure 1
Pathological examination of hepatocellular carcinoma. (A)
hepatocellular carcinoma (HCC) trabecular, solid and pseudo-glandular in
explanted cirrhotic liver (HE, × 40); (B) HCC trabecular and solid adjacent
regenerative nodule (HE, × 400).
Figure 2
Figure 3
Figure 3
Computer tomography of the chest showing pulmonary nodules
with soft tissue density measuring right up to 2.5 cm in diameter.
Figure 3
Figure 4
A Late pulmonary recurrence of hepatocellular carcinoma (A), and
solid and trabecular recurrent hepatocellular carcinoma (B). A: HE, × 100;
B: HE, × 400.
Discussion
Tumor recurrence after liver transplantation is “early” when it
occurs within 2 years, “late” when it occurs between 2 and 5 years and those that occur after 5 years of the cancer recovery are considered
“very late” tumor recurrences [1,5-7]. Most recurrences usually occur
within the first two years after treatment. The literature does not
contain many cases of very late recurrences.
Our patient received a liver transplant due to alcoholic cirrhosis
and HCC, which was followed by kidney transplantation six years
later. He had an HCC recurrence 8.5 years (101 months) after liver
transplantation.
During liver transplantation, factors associated with poor survival
were discovered: a multifocal tumor, poor differentiation and vascular
invasion, known as a predictive factor for tumor reappearance.
Although we cannot prove it, we can consider that the balance of these
immunosuppressive drugs may have played a role in the long-term
survival of HCC in our patient after his liver transplant. On the other
hand, we can also postulate that the over-immunosuppression after
his kidney transplant, with a triple regimen with ATG, mycophenolic
acid and a corticosteroid [8], may have been an important influence
on HCC recurrence at a very late time point.
The concept of immunosuppression as an important factor for
tumor growth was postulated by Yokoyama [9], who demonstrated
that the time for doubling the diameter of a recurrent tumor in a
transplanted liver under immunosuppression was only 37 days,
whereas the doubling of the tumor recurrence in un transplanted
cirrhotic patients without therapeutic immunosuppression occurred
in 273 days. Experimental studies also demonstrated that cyclosporine
A (CsA) increases the growth and invasiveness of tumor cells by
inhibiting DNA repair functions [10].
Clinical Studies from the Bologna Group have shown that the
cumulative dose of CSA in the first year after transplantation is an
important factor in tumor recurrence. They also analyzed two patient
groups transplanted because of HCC, one treated with cyclosporine
and the other with tacrolimus. As a high relapse rate was noted in
both groups, they recommend that calcineurin inhibitors should be
used with caution in patients transplanted for HCC [11].
Recent studies have demonstrated that elevated levels of
calcineurin inhibitors within the first month after transplantation, i.e.
tacrolimus > 10 ng/ml or cyclosporine > 300 ng/ml are associated with
increased relapse of HCC [12]. Another study with approximately
36,000 kidney transplant patients demonstrated a higher incidence
of cancer in this group of patients than in the general population and,
once more, immunosuppression was associated with a carcinogenic
effect [13].
S-adenosyl-L-methionine studies have suggested that sirolimus
and everolimus, both inhibitors of the mammalian target of rapamycin
(mTOR), are new immunosuppressant drugs with an antitumor
effect due to their ability to inhibit the proliferation of tumor cells.
It has also been demonstrated that sirolimus and everolimus are safe
and increase survival in liver transplant patients with HCC [14].
Geisseler et al. [15] showed that sirolimus in liver transplant
recipients with HCC do not improve long-term recurrence-free
survival beyond 5 years. However, a recurrence-free survival and
overall survival benefit was evident in the first 3 to 5 years, especially
in low-risk patients. This trial provided the first high-level evidence
for selecting an immunosuppression regime in liver transplant
recipients with HCC.
Both our patient and Schreibman’s patient had similar high risk factors for HCC recurrence, such as over-immunosuppression and
the timing of disseminated HCC recurrence after sequential liverkidney
transplantation, and both patients had a very low chance of
effective treatment. Also, the coincident time of HCC recurrence of
these two patients after sequential transplantation may focus attention
on using a less powerful immunosuppressive regimen after kidney
transplantation. The literature associates longer disease-free survival
with a milder immunosuppressive regimen [16]. Additionally, we can
also postulate about the benefit of screening the patient more often
for HCC recurrence after kidney transplantation, in anticipation to
the risk of severe disseminated disease on long-term follow-up.
In conclusion, we present the second case of HCC recurrence
after sequential liver and kidney transplantation with a combination
of risk factors such as tumor size, vascular invasion, overimmunosuppression
and double transplantation. Therefore, we can
suggest that patients with HCC who undergo to double sequential
transplant, during long-term follow-up, should receive the mildest
immunosuppressive regimen possible and more frequent screening
for HCC recurrence, i.e. at least every 6 months. We highlight the
need for further randomized studies to identify the best protocol for
immunosuppression in liver transplant patients with hepatocellular
carcinoma to prevent the recurrence of the tumor.
References
- Schreibman IR, Bejarano P, Martinez EJ, Regev A. Very Late Recurrence of Hepatocellular Carcinoma after Liver Transplantation: Case Report and Literature Review. Transplant Proc. Elsevier; 2006; 38: 3140-3143.
- Pérez-Saborido B, de los Galanes SJ, Menéu-Díaz JC, Romero CJ, Elola- Olaso AM, Suárez YF, et al. Tumor Recurrence After Liver Transplantation for Hepatocellular Carcinoma: Recurrence Pathway and Prognostic Factors. Transplant Proc. Elsevier; 2007; 39: 2304-2307.
- Cheng J-W, Shi Y-H, Fan J, Huang X-W, Qiu S-J, Xiao Y-S, et al. An immune function assay predicts post-transplant recurrence in patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. Springer-Verlag. 2011; 137: 1445-1453.
- Raza A, Sood GK. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol. 2014; 20: 4115-4127.
- Castroagudín JF, Molina-Pérez E, Ferreiro-Iglesias R, Abdulkader I, Otero- Antón E, Tomé S, et al. Late Recurrence of Hepatocellular Carcinoma after Liver Transplantation: Is an Active Surveillance for Recurrence Needed? Transplant Proc. Elsevier. 2012; 44: 1565-1567.
- Chan AC, Poon RT, Cheung TT, Chok KS, Chan SC, Fan ST, et al. Survival analysis of re-resection versus radiofrequency ablation for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. World J Surg. 2012; 36: 151-156.
- Nelson AC, Jessurun J, Peterson RK, Pambuccian SE. Intrahepatic recurrence of hepatocellular carcinoma 13 years after orthotopic liver transplantation for hepatitis C-related cirrhosis with occult hepatocellular carcinoma: A case report. Liver Transplant. Wiley Subscription Services, Inc., A Wiley Company. 2012; 18: 612-614.
- Miyagi S, Kawagishi N, Sekiguchi S, Akamatsu Y, Sato K, Takeda I, et al. The Relationship Between Recurrences and Immunosuppression on Living Donor Liver Transplantation for Hepatocellular Carcinoma. Transplant Proc. Elsevier. 2012; 44: 797-801.
- Yokoyama I, Carr B, Saitsu H, Iwatsuki S, Starzl TE. Accelerated growth rates of recurrent hepatocellular carcinoma after liver transplantation. Cancer. 1991; 68: 2095-2100.
- Schlitt HJ, Mornex F, Shaked A, Trotter JF. Immunosuppression and hepatocellular carcinoma. Liver Transplant. Wiley Subscription Services, Inc., A Wiley Company. 2011; 17: S159-S161.
- Vivarelli M, Cucchetti A, La Barba G, Ravaioli M, Del Gaudio M, Lauro A, et al. Liver transplantation for hepatocellular carcinoma under calcineurin inhibitors: reassessment of risk factors for tumor recurrence. Ann Surg. 2008; 248: 857-862.
- Rodríguez-Perálvarez M, Tsochatzis E, Naveas MC, Pieri G, García- Caparrós C, O’Beirne J, et al. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J Hepatol Elsevier. 2013; 59: 1193-1199.
- Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004; 4: 905-913.
- Liang W, Wang D, Ling X, Allen Kao A, Kong Y, Shang Y, et al. Sirolimusbased immunosuppression in liver transplantation for hepatocellular carcinoma: A meta-analysis. Liver Transplant. Wiley Subscription Services, Inc., A Wiley Company. 2012; 18: 62-69.
- Geissler EK, Schnitzbauer AA, Zülke C, Lamby PE, Proneth A, Duvoux C, et al. Sirolimus Use in Liver Transplant Recipients With Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation. 2016; 100: 116-125.
- Toso C, Cader S, Mentha-Dugerdil A, Meeberg G, Majno P, Morard I, et al. Factors predicting survival after post-transplant hepatocellular carcinoma recurrence. J Hepatobiliary Pancreat Sci. 2013; 20: 342-347.