Case Report
Clinicopathological Study of Eight Cases of Gastric Neuroendocrine Carcinoma
Keishiro Aoyagi*, Junya Kizaki, Taro Isobe, Taizan Minami and Yoshito Akagi
Department of Surgery, Kurume University School of Medicine, Japan
*Corresponding author: Keishiro Aoyagi, Department of Surgery, Kurume University School of Medicine, 67 Asahi-machi, Kurume, Fukuoka 830-0011, Japan
Published: 02 Dec, 2016
Cite this article as: Aoyagi K, Kizaki J, Isobe T, Minami T,
Akagi Y. Clinicopathological Study of
Eight Cases of Gastric Neuroendocrine
Carcinoma. Ann Clin Case Rep. 2016;
1: 1197.
Abstract
Neuroendocrine carcinoma (NEC) of the stomach accounts for 0.1% to 0.6% of all gastric carcinomas, and its prognosis is poor. This report describes eight patients with NEC of the stomach. Only one patient had been correctly diagnosed before surgery; the others had been misdiagnosed with tubular adenocarcinoma, poorly differentiated adenocarcinoma, and malignant lymphoma. Five patients had type 2 cancer on macroscopic examination. Histological findings of the resected specimens showed that three NECs were associated with tubular adenocarcinoma and that one was associated with signet ring cell carcinoma. Liver metastases were found in four patients, but none had peritoneal metastases. The cancer stroma volume and tumor infiltration indicated the medullary type in seven patients and expanding growth type in six. Neither the scirrhous type nor infiltrative growth type was found. All eight patients had moderate or marked lymphatic invasion, and six had venous invasion. Six patients underwent postoperative chemotherapy. The median survival time was 10 months, and the 5-year survival rate was 37.5%. The causes of death were liver metastases in five patients and metastases to the lung and brain in one. One patient with lung metastasis who underwent multimodal treatment comprising surgery, radiation, and chemotherapy with cisplatin + irinotecan and S-1 + paclitaxel remained alive for 74 months postoperatively. NEC was difficult to diagnose preoperatively. High frequencies of capillary invasion and hematogenous metastasis, such as to the liver, were observed, and the prognosis was poor. However, long-term survival was higher among patients with NEC who underwent multimodal therapy.
Introduction
The World Health Organization classification of 2010 defined neuroendocrine carcinoma
(NEC) as a subgroup of neuroendocrine neoplasms. Neuroendocrine neoplasms are classified as
neuroendocrine tumors or NECs according to their bioactivity, which is determined by the mitotic
rate and Ki67 index (Table 1) [1]. The Japanese classification of gastric carcinoma defines NEC as a special type in the histological classification of gastric tumors and considers NEC to be either small
cell type or large cell type [2].
NECs are characterized by many mitoses (≥20) and high proliferative activity (Ki67 index
of ≥20%) (Table 1) [1]. Many NECs show medullary, expanded, and trabecular proliferation,
and rosette structures are seen pathologically [3,4]. Argyrophilic granules, which are stained by Grimelius stain, or argentaffin granules, which are stained by Fontana–Masson stain, are detected
in the cytoplasm of NEC cells. Neuroendocrine granules are seen in the cytoplasm of NEC cells
on electron microscopy. NECs are positive for neuroendocrine markers such as chromogranin
A, synaptophysin, and neural cell adhesion molecule (NCAM/CD56) on immunohistochemical
staining [5].
NEC of the stomach is relatively rare,
accounting for 0.1% to 0.6% of all gastric carcinomas [6,7]. It has a high frequency of capillary invasion, lymph node metastasis, and hematogenous metastasis,
such as to the liver and lung. Its prognosis is poor [3,4].
The details of eight cases of NEC of the stomach resected in our institute are herein presented.
Case Presentation
Patients
From 1994 to 2013, 2559 patients with histologically confirmed primary gastric cancer underwent
surgery at the Department of Surgery, Kurume University School of Medicine, Kurume, Japan.
During this time, eight patients (0.31%) were diagnosed with NEC of the stomach. All eight patients
were male, and their mean age was 69.6 years (range, 64-76 years). All eight patients underwent clinicopathological examination. The clinicopathological terms used
were those outlined in the 3rd English edition [2] and 14th Japanese
edition [5] of the Japanese Classification of Gastric Carcinoma.
This study was conducted in accordance with the Declaration of
Helsinki and approved by the Research Ethics Committee of Kurume
University School of Medicine. The requirement for informed
consent was waived because of the retrospective study design.
Special staining
Grimelius staining was performed for four patients.
Immunohistochemical staining was performed using chromogranin
A antibody for seven patients, synaptophysin antibody for three patients, CD56 antibody for three patients, and neuron-specific
γ-enolase (NSE) antibody for four patients.
Ki67 index
Ki67 staining was performed immunohistochemically for all eight
patients. Ki67-positive cancer cells per 1000 cancer cells were counted
in 10 high-power fields (HPF), and the Ki67 index (%) was calculated.
Mitotic count
Cancer cells with mitoses were counted in 10 HPF.
Survival
Overall survival for all eight patients was calculated according to
the Kaplan–Meier method.
Table 1
Table 2
Table 3
Table 4
Table 5
Table 6
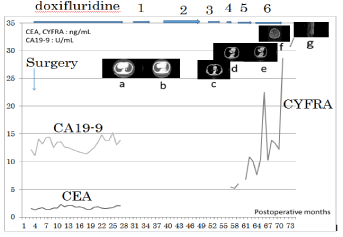
Figure 1
Figure 1
CDDP (90 mg, day1) + CPT-11 (days 1, 8, 15) (28-day cycle) +
radiation 45 Gy : 6 courses , 2: S-1 (80 mg, days 1-14) + PTX (70 mg, days
1, 8) (21-day cycle) : 9 courses, 3: amrubicin (60 mg days 1-3) (21-day cycle)
: 6 courses, 4: CBDCA (280 mg, day 1) + etoposide (130 mg, days 1-3)
(21-day cycle) : 4 courses, 5: nogitecan (1.1 mg, days 1-5) (28-day cycle) :
6 courses, 6: docetaxel (70 mg, day 1) (21-day cycle) : 8 courses a: Mass of
recurrence, 20 mm in size was recognized at S10 of the left lung. b: Chest CT
revealed no mass at S10 of the left lung. c: Swelling of a lymph node 20mm in
size, is recognized at the hilum of the left lung. d: Chest CT showed that the
mass lesion at S10 increased to 46 x 30 mm in size. e: Chest CT revealed
that the mass lesion at S10 increased to 65 x 40 mm, and a small amount
of effusion had developed. f: Brain MRI revealed multiple mass lesion with
ring-like enhancement. g: MRI of vertebrae showed bone metastases in the
thoracic vertebrae.
Results
Clinicopathological study
All eight patients were symptomatic on admission. One patient
had early cancer, but the other patients had advanced cancer (Table
2). Before surgery, only one patient was diagnosed with NEC, five
patients were diagnosed with poorly differentiated adenocarcinoma,
and two patients were diagnosed with tubular adenocarcinoma. Two
patients were diagnosed with malignant lymphoma on preoperative
diagnostic imaging (Table 3). Five patients had type 2 cancer, and the
NECs of four patients occurred in the lower third (L) of the stomach.
A 65-year-old man (Case 3) who underwent distal gastrectomy for a
duodenal ulcer 35 years earlier had an NEC arising from the gastric
suture line of his remnant stomach. A 64-year-old man (Case 4) had two gastric cancer lesions; one was type 2 cancer with an NEC in the
L region with duodenal invasion, and the other was a 0-IIc lesion
with a histological diagnosis of tub1 in the upper third part (U) of
the stomach. At the time of surgery, seven patients had lymph node
metastases, five patients had stage T4a or T4b cancer, and four patients
had liver metastases. A 74-year-old man (Case 8) had simultaneous
liver, lung, and distant lymph node metastases. However, no patients
had peritoneal metastases (Table 2).
Histological examination revealed that five patients had small
cell carcinoma and three patients had large cell carcinoma. All
patients had moderate or marked lymphatic invasion (ly2 or ly3),
and six patients had venous invasion. Seven patients had medullary
type NEC, and no patients had scirrhous type NEC. Six patients had
expanding growth (INFa), and no patients had infiltrative growth
(INFc). Case 1 showed medullary and irregular infiltration of tumor
cells with little cytoplasm and round or spindle-shaped chromatinrich
nuclei. Some of the tumor cells formed a glandular or trabecular
pattern, and necrotic tissue was seen in some areas. In Case 6, the
tumor cells were arranged in a sheet formation, and many mitoses
were seen. The mean major axis of the tumors was 60.0 mm (range,
25-90 mm). Well-differentiated tubular adenocarcinoma was seen
in the superficial region in three patients. A 67-year-old man (Case
7) showed non medullary infiltration and signet ring cell carcinoma
in the superficial region, with distribution of intramural metastases
throughout the entire stomach; this patient remained alive without
recurrence for 72 months after surgery (Table 4). The mean number
of cancer cells with mitoses in 10 HPF was 102.8 (range, 32–183 per 10
HPF). The mean Ki67 index was 37.8% (range, 23.1%–80.5%). Four
tumors were positive on Grimelius staining, and four were positive
for NSE. Three tumors were positive for synaptophysin. Six tumors
were positive and one was negative for chromogranin. Two tumors
were positive and one was negative for CD56 (Table 5).
Tumor markers
Carcinoembryonic antigen was upregulated in two patients
and not upregulated in six patients. Carbohydrate antigen 19-9
was upregulated in one patient and not upregulated in seven
patients. Cancer antigen 72-4 was upregulated in one of six patients
tested. NSE was upregulated in three of four patients tested. Both
carcinoembryonic antigen and carbohydrate antigen 19-9 were
highly upregulated in a 74-year-old man (Case 8) who had liver, lung,
and distant lymph node metastases and died 1 month after surgery.
Although cytokeratin 19 fragment is a sensitive marker for non-small
cell lung cancer, it was a very sensitive marker for progression and
metastases in a 69-year-old man (Figure 1) (Table 6).
Surgery
Three patients underwent distal gastrectomy. Four patients
underwent total gastrectomy. One patient underwent resection of
the remnant stomach. On lymph node dissection (D), one patient
was D1, two were D1+, and five were D2. Four patients underwent
curative resection, and four underwent non curative resection due to
liver or liver + distant metastases (lung and para-aortic lymph node metastases) (Table 4).
Chemotherapy and radiation
Six patients underwent chemotherapy for metastases or
postoperative adjuvant chemotherapy. Two patients did not undergo
chemotherapy because they either refused or had a poor general
condition due to rapid tumor growth. A 71-year-old man (Case 1)
who refused postoperative chemotherapy remained alive for 124
months without recurrence. A 76-year-old man (Case 2) with early
cancer did not undergo postoperative chemotherapy but underwent
chemotherapy after liver recurrence. Four patients underwent
intrahepatic arterial infusion (IHA) using 10 or 20 mg of cisplatin
(CDDP) for liver metastases. A 67-year-old man (Case 7) underwent
postoperative adjuvant therapy only. A 71-year-old man (Case 5)
required third-line chemotherapy for liver metastases including IHA
using CDDP, irinotecan (CPT-11), and paclitaxel (PTX) (Table 2).
A 69-year-old man (Case 6) underwent several chemoradiotherapy
regimens, including CDDP + CPT-11 + radiation, S-1 + PTX,
amrubicin (AMR), carboplatin (CBDCA) + etoposide (ETP),
nogitecan (NGT), and docetaxel (DOC), for metachronous lung
metastases and radiation for brain and bone metastases for 43
months. He finally died of brain metastases 74 months after surgery
(47 months after recognition of the lung metastases) (Table 2) (Figure
1).
Prognosis
The median overall survival time was 10 months (range, 1–124
months). The 5-year survival rate was 37.5%. Six patients died of
recurrence and metastasis of NECs, and two patients were alive
without recurrence for more than 5 years after surgery. A 69-year-old
man (Case 6) with metachronous lung metastases at 27 months after
surgery survived for 74 months with multimodal therapy including surgery, chemotherapy, and radiation. The cause of death was liver
metastasis in five patients and brain metastasis in one. A 76-year-old
man (Case 2) with early gastric cancer died of metachronous liver
metastases.
Discussion
Iwafuchi et al. [8] described four pathogenetic pathways of
stomach NEC: from common-type adenocarcinoma, from a
carcinoid tumor, from multipotential stem cells, and from immature
neuroendocrine cells. Many NECs of the stomach have recently been
considered to originate from common-type adenocarcinoma. The
cell clone of the endocrine cell carcinoma is believed to originate
from intramucosal adenocarcinoma [8]. Differentiated tubular
adenocarcinoma is thought to be particularly significant for the
occurrence of endocrine cell carcinoma because well-to-moderately
differentiated tubular adenocarcinoma is frequently seen in the
superficial regions of the stomach in many patients with NEC [9].
In the present study, well-differentiated tubular adenocarcinoma
was seen in the superficial region of the stomach in three patients,
and Case 7 showed non medullary infiltration and signet ring cell
carcinoma in the superficial region of the stomach. Iino et al. [10] reported that the biological characterization of NEC depended on
the histological type of adenocarcinoma from which it was generated
or the component of adenocarcinoma that was dedifferentiated with
growth of the NEC. In Case 7, signet ring cells were seen and the NEC
cells showed infiltrative growth [11].
More than 50% of NECs are localized in the lower third of the
stomach; approximately 80% are macroscopic type 2, and many
NECs show medullary infiltration and expanded proliferation [3,4].
These features were found in the present study.
NEC has a poor prognosis and is characterized by a high frequency
of capillary invasion, lymph node metastasis, and hematogenous
metastasis, such as to the liver and lung, either intraoperatively or
in the early postoperative phase [8,9]. Nishikura et al. [9] reported that 75% of gastric NECs were advanced cancers and that 83% of
gastric NECs had capillary invasion. Iwafuchi et al. [8] reported that
capillary invasion was detected in 82.4% of 17 early gastric NECs. In
the present study, all patients had moderate or marked lymphatic
invasion (ly2 or ly3), six patients had venous invasion, and seven
patients had advanced cancer. Hematogenous metastases such as to
the liver and/or lung occurred in six patients, including one with early
gastric NEC.
Establishment of a definitive diagnosis of gastric NEC before
surgery was difficult in the present study because many gastric NECs
mainly develop in the submucosal layer. Only one patient in the
current study was diagnosed with NEC before surgery. The differential
diagnoses included carcinoid, malignant lymphoma, undifferentiated
carcinoma, metastatic carcinoma from small cell lung cancer; solidtype
poorly differentiated adenocarcinoma, and poorly differentiated
squamous cell carcinoma [8].
NSE is reportedly a sensitive tumor marker for neuroendocrine
tumors [12]. In the present study, NSE was upregulated in three of
four patients. All four patients who underwent immunohistochemical
staining for NSE showed positive results.
With respect to the treatment of gastric NEC, the National
Comprehensive Cancer Network guideline recommends multimodal
therapy including surgical resection of the stomach and postoperative
administration of a regimen for small cell lung cancer, such as CDDP
+ ETP, CDDP + CPT-11, or CBDCA + ETP [13]. S-1 alone or S-1 +
CDDP has been administered as the standard treatment for advanced
gastric cancer [14]. Additionally, several case reports documented
good clinical effects of S-1 or S-1 + CDDP for NEC of the stomach
in Japan [15-17]. Hainsworth et al. [18] performed a phase II trial of three anticancer drugs including taxane (PTX, CBDCA, and ETP) in
patients with poorly differentiated NEC of the gastrointestinal tract
and reported a response rate of 53% and median progression-free
survival time of 14.5 months. Five patients underwent IHA using
CDDP for liver metastases. However, in these patients who underwent
IHA, IHA was not thought to be effective for liver metastasis of
gastric NECs. Case 6 underwent several chemoradiotherapy regimens
including CDDP + CPT-11 + radiation, S-1 + PTX, AMR, CBDCA +
ETP, NGT, and DOC for metachronous lung metastasis and radiation
for brain and bone metastases for 43 months. He finally died of brain
metastases 74 months after surgery (47 months after recognition
of the lung metastases). While this patient’s first regimen included
CDDP + CPT-11 + radiation and the clinical effect was a complete
response, a single metastatic nodule was localized to S10 of the left
lung; thus, radiation was thought to be effective as local therapy for
this metastatic nodule. Moreover, radiation was performed for brain
and bone metastases and was thought to be useful for prolonging
survival and improving the patient’s quality of life. This case suggests
that the addition of S-1 and taxane to a small cell lung cancer regimen
with radiation prolongs the survival of patients with recurrent NEC
of the stomach [19]. In the present study of patients with gastric NEC,
we recommended resection of the stomach including the metastatic
site. Chemotherapy for small cell lung cancer was also needed as
postoperative adjuvant treatment. Multimodal therapy including
surgery, chemotherapy, and radiation was needed for management of
inoperable and recurrent NEC.
The prognosis of gastric NEC was poor in the present study. In
previous studies, the 5-year survival rate and median survival time of
patients with gastric NEC were reportedly 24.2% and 7 to 9 months,
respectively [20,21]. In our study, the median overall survival time
was 10 months (range, 1-124 months), and the 5-year survival rate
was 37.5%. The cause of death was liver metastasis in five patients,
including one with early gastric cancer, and brain metastasis in one
patient. The 5-year survival rate of our patients was better than that in
previous reports. However, the median survival time of our patients
was almost the same as that in previous reports.
Conclusion
In this study, NEC was difficult to diagnose preoperatively. High frequencies of capillary invasion and hematogenous metastasis, such as to the liver, were seen in our patients with NEC, and the prognosis was poor. Even early NEC was thought to require postoperative adjuvant chemotherapy. However, patients with NEC who underwent multimodal therapy had longer-term survival.
Acknowledgments
The authors express their sincere appreciation to Professor Masayoshi Kage of the Department of Diagnostic Pathology, Kurume University Hospital for providing assistance with interpretation of the pathological findings and performing the immunohistochemical workup.
References
- Rind G, Arnold R, Bosman FT, Capella C, Klimstra DS, Klöppel G, et al. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumors of digestive system. 4th ed. Lyon: WHO; 2010. 13-14.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011; 14: 101-112.
- Haraoka S, Ikeda K, Tanabe H, Ota A, Iwashita A. Carcinoid and endocrine carcinoma of the stomach: from the standpoint of pathologist (in Japanese with an English abstract). Stomach Intestine. 2010; 45: 1894-1905.
- Nimura S, Sato K. Macroscopic and microscopic pathology of special types of gastric carcinoma (in Japanese with an English abstract). Stomach Intestine. 2010; 45: 1870-1881.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 14th Japanese edition. Tokyo: Kanehara; 2010.
- Matsusaka T, Watanabe H, Enjoji M. Oat-cell carcinoma of the stomach. Fukuoka Acta Med. 1976; 67: 65-73.
- Watanabe H, Jass JR, Sobin LH. Histological typing of oesophageal and gastric tumors. 2nd ed. Berlin: Springer-Verlag; 1990; 19-28.
- Iwafuchi M, Watanabe H, Ishihara N, Noda H, Ajioka Y. Pathology of carcinoid and endocrine carcinoma of the digestive tract: their features and carcinogenesis (in Japanese). Clin Gastroenterol. 1990; 5: 1669-1681.
- Nishikura K, Watanabe H, Iwafuchi M, Fujiwara T, Kojima K, Ajioka Y. Carcinogenesis of gastric endocrine cell carcinoma: analysis of histopathology and p53 gene alteration. Gastric Cancer. 2003; 6: 203-209.
- Iino I, Sakaguchi T, Ohta M, Kamiya K, Baba S, Konno H. A case of neuroendocrine carcinoma of the stomach with invasion to the pancreas (in Japanese with an English abstract). J Jpn Coll Surg. 2012; 37: 784-789.
- Aoyagi K, Kizaki J, Isobe T, Akagi Y. A case of gastric cancer with neuroendocrine carcinoma, signet ring cell carcinoma components, and intramural metastases. Am J Case Rep. 2016; 17: 274-279.
- Lindholm DP, Oberg K. Biomarkers and molecular imaging in gastroenteropancreatic neuroendocrine tumors. Horm Metab Res. 2011; 43: 832-837.
- NCCN clinical practice guidelines in oncology neuroendocrine tumors v.1. 2011 [cited 22 July 2016].
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines. 2010 (ver. 3). Gastric Cancer. 2011; 14: 113-123.
- Hanada N, Inoue K, Osako T, Kuriwaki K, Shimajiri S, Mochinaga M. A case of partial response in liver metastatic lesion from gastric endocrine cell carcinoma treated with TS-1 (in Japanese with English abstract). Jpn J Cancer Chemother. 2006; 33: 1143-1146.
- Kaneko S, Itsui Y, Hashiguchi M, Watanabe M, Yoshida R, Akashi T, et al. A case of gastric neuroendocrine carcinoma with liver metastasis and portal vein invasion successfully treated by S-1 and cisplatin chemotherapy (in Japanese with English abstract). J Jpn Soc Gastroenterol. 2013; 110: 56- 63.
- Iwasaki K, Katayanagi S, Sumi T, Tsuchida A, Kawachi S, Shimazu M. A review of 6 cases of endocrine carcinoma of the stomach (in Japanese with English abstract). J Jpn Surg Assoc. 2014; 75: 2962-2966.
- Hainsworth JD, Spigel DR, Litchy S, Greco FA. Phase II trial of paclitaxel, carboplatin, and etoposide in advanced poorly differentiated neuroendocrine carcinoma: a Minnie Pearl Cancer Research Network Study. J Clin Oncol. 2006; 24: 3548-3554.
- Aoyagi K, Kizaki J, Isobe T, Akagi Y. Long-term survival of a patient with small cell carcinoma of the stomach with metachronous lung metastases treated by multimodal therapy: a case report. Surg Case Rep. 2015; 1: 125.
- Hibi S, Terasaki M, Okamoto Y, Goto Y, Kurumiya Y, Shingu Y, et al. A case of endocrine cell carcinoma with adenocarcinoma and the analysis of 71 cases reported in Japan (in Japanese with English abstract). Jpn J Cancer Clin. 2002; 48: 807-812.
- Kuramoto M, Hasuo T, Ishihara K, Ikeshima S, Iwatsuki M, Shimada S. A case of gastric small cell carcinoma (in Japanese with English abstract). J Jpn Surg Assoc. 2005; 66: 2436-2440.