Case Report
Autoimmune Hepatitis-Induced Cirrhosis due to Long-Term Methotrexate Therapy for Rheumatoid Arthritis
Habeeb Salameh1, Jack Johnson2, Kaled Diab2, Hamzeh Saraireh1, Shehzad N. Merwat1 and Heather L. Stevenson3*
1Department of Internal Medicine, University of Texas Medical Branch at Galveston, USA
2School of Medicine, University of Texas Medical Branch, USA
3Department of Pathology, University of Texas Medical Branch at Galveston, USA
*Corresponding author: Heather L. Stevenson, Department of Pathology, University of Texas Medical Branch at Galveston, 301 University Blvd 77555, Galveston, TX, USA
Published: 09 Sep, 2016
Cite this article as: Salameh H, Johnson J, Diab K,
Saraireh H, Merwat SN, Stevenson
HL. Autoimmune Hepatitis-Induced
Cirrhosis due to Long-Term
Methotrexate Therapy for Rheumatoid
Arthritis. Ann Clin Case Rep. 2016; 1: 1122.
Abstract
Methotrexate (MTX) is commonly used to treat rheumatoid arthritis and can adversely affect the liver. Autoimmune hepatitis (AIH) is an extremely rare manifestation of MTX induced liver disease and has only been described in two previous cases. This report describes a patient that developed AIH, incomplete cirrhosis and portal hypertension after 10 years of MTX therapy. Liver function improved after discontinuation of MTX and proper treatment. Internists, family practitioners, gastroenterologists and hepatologists should be aware of the uncommon side effects of MTX and appropriately monitor patient’s liver function tests. This will lead to earlier detection and prevention of bad clinical outcomes.
Keywords: Methotrexate (MTX); Autoimmune hepatitis; Rheumatoid arthritis; Liver cirrhosis
Introduction
Methotrexate (MTX) is commonly used to treatrheumatoid arthritis (RA) [1]. Abnormal liver
function tests occur commonly with MTX, but serious hepatotoxicity is rare [2,3]. Therefore, liver
function tests should be performed regularly to assess for hepatic injury.
Autoimmune hepatitis (AIH) is a chronic inflammatory liver disease primarily seen in young
women [4]. The exact etiology and pathogenesis is unknown but is likely due to environmental
factors and/or loss of immune tolerance in genetically susceptible hosts. Most cases are idiopathic
with an unknown etiology. However, some known environmental triggers include medications,
herbal agents, and viral hepatitis [4]. Herein, we report a MTX treated patient with RA who
subsequently developed AIH that remained undetected until she developed incomplete cirrhosis
and portal hypertension.
Case Presentation
We describe the case of a 66 year old African American female with a past medical history of
being overweight (body mass index of 27.4 kg/m),having longstanding type-2 diabetes mellitus of
45 years duration, chronic kidney disease diagnosed 2 years prior to presentation, and anemia of
chronic disease. In 2002 she was also diagnosed with erosive, non-deforming rheumatoid arthritis
involving her bilateral wrists, elbows and knees. At that time she was prescribed MTX 7.5 mg weekly
along with a folic acid supplement. While previously she had normal liver function tests, her liver
enzymes (ALT, AST, and alkaline phosphatase) started to show intermittent mild elevations of
less than 2 times the upper limit of normal between 2009 and 2011. She never consumed alcohol
and her medications included metformin, folic acid, lisinopril, isosorbide dinitrate, nitroglycerin,
mirtazapine and MTX (cumulative dose of 2.73 grams). Her viral serologies were negative but no
further work up was pursued. She presented to the emergency department in July of 2012 with a
3-week history of abdominal swelling, abdominal pain, nausea, vomiting, weight loss, and fatigue.
She reported her father had liver disease but further details were not known. Physical exam revealed
as cites and splenomegaly. Abdominal ultrasound showed as cites, heterogeneous liver echogenicity
and nodular contour, suggestive of cirrhosis. Her initial laboratory studies including an autoimmune
liver disease panel are outlined in Table 1. Her Model for End-Stage Liver Disease (MELD) score
was 14, which was primarily due to a creatinine of 1.5 mg/dl and an INR of 1.4. Her Child-Turcotte-
Pugh score was class B. Ferritin levels were elevated at 1130 ng/mL and ceruloplasmin was normal
at 36 mg/dL. Repeat viral serologies were negative.
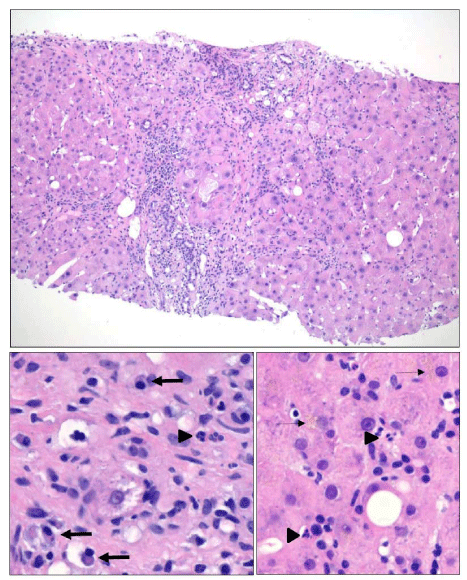
A liver biopsy was performed and histology is illustrated in
(Figure 1). The biopsy showed chronic hepatitis with prominent
interface activity, moderate portal lymphoplasmacytic portal
inflammation, and frequent plasma cells were observed throughout
the lobules. The modified hepatitis activity index (MHAI) was
10/18 and the Ishak fibrosis stage was 5/6, which was confirmed
with a Masson’s trichrome stain (data not shown) [5]. The biopsy
also showed focal macrovesicular steatosis (involving less than 5%
of hepatocytes), minimal bile ductular reaction, and there was no
evidence of infiltrative disease, such as amyloidosis. The iron stain
on the biopsy was negative indicating that the elevated ferritin was
not associated with hepatic hemosiderosis. The AIH diagnostic score
using the revised original scoring system [6] was 16 (>15= definite
AIH) and the diagnostic score using the simplified scoring system
[7] was 7 (≥6 = definite AIH).AIH was diagnosed and MTX therapy
was subsequently discontinued. Treatment with prednisone (30 mg
taper; decreased to 10 mg by week 4) and azathioprine (50 mg) was
initiated. The latter was continued until 2015.
After four years her laboratory studies were repeated and are
outlined in Table 1. Between her initial presentation and followup,
she was diagnosed with end-stage renal disease secondary to
uncontrolled diabetes. Her MELD score was 21due to a creatinine
of 3.3 mg/dl and an INR of 1.1. She was now a Child-Turcotte-
Pugh class A. The repeated autoimmune laboratory studies showed
negative anti-nuclear antibodies (ANA), negative alpha-smooth
muscle actin (ASMA) antibodies, borderline positive anti-F-actin
antibodies, negative anti-LKM-1 antibodies, and normal IgG
immunoglobulin levels. Her current sequelae of portal hypertension
include thrombocytopenia and esophageal varices. According to the
above AIH scoring systems; she no longer qualified for a diagnosis of
AIH [6].
Discussion
Before considering drug-induced liver injury in patients
presenting with elevated liver enzymes, a thorough work-up should
rule out other etiologies including primary biliary cirrhosis, primary
sclerosing cholangitis, and occult HBV or HCV infections, which
we conduct in all of our patients. While lisinopril has been reported
to be associated with cholestatic liver injury [8], none of her other
medications have been reported to be associated with drug-induced
liver injury or drug-induced auto-immune hepatitis. Furthermore,
liver biopsy did not show typical findings of steatosis, non-alcoholic
steatohepatitis, amyloidosis, or glycogen hepatopathy. Differentiation
between true AIH and drug-induced AIH remains a diagnostic
challenge [9]. A recent study found that most cases of drug-induced
liver injury are attributed to nitrofurantoin or minocycline and that
induced by methyldopa and hydralazine resembles auto-immune
hepatitis in almost half of the cases [10].
MTX-induced liver injury has been reported to occur in patients
treated for leukemia, psoriasis, rheumatoid arthritis and a variety of
other diseases [11-16]. Transaminase elevation is the most frequently
described hepatotoxicity [17]. Less common but significantly more
dangerous side effects include hepatic steatosis, fibrosis, and even
cirrhosis [18-20]. Kremer et al. [21] reported that patients with
elevated liver enzymes due to MTX respond to dose adjustments,
which reduced the likelihood of development of clinically significant
liver disease. Paradoxically, MTX has been used successfully to treat
AIH Type 1 that is refractory to standard therapy [22]. Another
report described two pediatric patients who presented with steroiddependent
AIH disease that was refractory to 6-Mercaptopurine
(6MP)/azathioprine (AZA) maintenance therapy that were
successfully treated with MTX [23].
AIH is an extremely rare manifestation of drug-induced liver
injury secondary to MTX and to our knowledge, has only been reported
twice in the literature. The two previous cases are outlined in Table
2 [24,25]. Classically, AIH is characterized by elevated AST, ALT,
IgG immunoglobulins, positive serum autoantibodies (ASMA/anti-
F-actin), and prominent interface hepatitis with lymphoplasmacytic inflammation by histology [7,26]. It is difficult to determine if AIH
is idiopathic or drug-induced based on the histopathologic features
alone; however, Suzuki et al. [27] reported that the presence of portal
neutrophils and cholestasis are more commonly observed in druginduced
AIH, and these features were observed in our patient’s
liver biopsy (Figure 1). The diagnosis of drug-induced AIH is best
confirmed by its resolution after removal of the inciting agent and the
lack of requirement for long-term steroid therapy [28].
The most common autoantibodies seen in AIH are ASMA,
anti-F-actin, ANA and anti-LKM-1. Our patient had positive ANA
and anti-F-Actin antibodies with negative ASMA and anti-LKM-1
antibodies. F-Actin is a component of the smooth muscle complex
and has been shown to be more sensitive and more specific for the
diagnosis of AIH when compared to ASMA [29]. Thus, even though
ASMA serology was negative, the diagnosis of AIH can still be made by the presence of positive anti-F-Actin antibodies.
The presence of incomplete cirrhosis and portal hypertension in
our patient resulted in abnormally low liver enzymes when compared
to the classic presentation of AIH. As hepatocytes are lost, the liver
loses its ability to synthesize proteins, such as albumin, transaminases,
and clotting factors [30]. At least a third of patients with AIH already
have cirrhosis at presentation, indicating the disease has progressed
unrecognized for a period of time prior to diagnosis [31]. In
hindsight, she started having intermittent, mild elevations in her liver
injury tests approximately 7 years after starting MTX, at which time
she had an incomplete work-up that was negative for infection with
hepatotropic viruses (i.e., HAV, HBV, and HCV).
After resolution of AIH, our patient’s albumin level increased, but
still remained slightly below normal, and her hepatic transaminases
have remained within the normal range for more than four years.
Since withdrawing MTX treatment, her autoantibodies have been
undetectable or within the normal range, her liver enzymes have
remained normal, and her hepatic synthetic function has improved,
which strongly supports that this medication was the inciting agent in
our case of drug-induced AIH [32]. A follow up liver biopsy was not
performed, as there was no clinical indication.
In summary, patients on long-term MTX therapy should be
monitored for the development of AIH by monitoring liver enzymes.
If patients are noted to have abnormal elevations, a further workup,
including laboratory studies for AIH should be considered. If
serologic studies are suggestive of AIH, a liver biopsy may be indicated
for confirmation and determination of fibrosis stage. Clinicians
need to be aware of the potential side effects of MTX therapy and
should appropriately monitor patients for drug-induced liver injury,
including AIH, which will lead to earlier detection and prevention of
poor clinical outcomes.
Figure 1
Figure 1
The liver biopsy showed moderate lymphoplasmacytic portal
inflammation with frequent neutrophils (arrow heads), prominent interface
and lobular inflammatory activity with abundant plasma cells (thick arrows),
hepatocyte rosette formation (all panels), and mild hepatocellular cholestasis
(thin arrows). The modified hepatitis activity index score was 10/18. Frequent
bridging fibrosis, areas of subsinusoidal fibrosis and focal nodule formation
were also observed. These features are consistent with incomplete cirrhosis
(Ishakfibrosis stage: 5/6).
Author Contribution
J Johnson, K Diaband H Saraireh wrote the manuscript and reviewed the literature. H Salameh, S Merwat and H.L. Stevenson critically revised the manuscript for important intellectual content, supervised the process and approved the final draft.
References
- Weinblatt ME, Coblyn JS, Fox DA, Fraser PA, Holdsworth DE, Glass DN, et al. Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med. 1985; 312: 818-822.
- Lewis JH, Schiff E. Methotrexate-induced chronic liver injury: guidelines for detection and prevention. The ACG Committee on FDA-related matters. American College of Gastroenterology. Am J Gastroenterol. 1988; 83: 1337-1345.
- Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis. 2009; 68: 1100-1104.
- Makol A, Watt KD, Chowdhary VR. Autoimmune hepatitis: a review of current diagnosis and treatment. Hepat Res Treat. 2011; 390916: 11.
- Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995; 22: 696-699.
- Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999; 31: 929-938.
- Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008; 48: 169-176.
- Hagley MT, Hulisz DT, Burns CM. Hepatotoxicity associated with angiotensin-converting enzyme inhibitors. Ann Pharmacother. 1993; 27: 228-231.
- Castiella A, Zapata E, Lucena MI, Andrade RJ. Drug-induced autoimmune liver disease: A diagnostic dilemma of an increasingly reported disease. World J Hepatol. 2014; 6: 160-168.
- de Boer YS, Kosinski AS, Urban TJ, Zhao Z, Long N, Chalasani N, et al. Features of Autoimmune Hepatitis in Patients With Drug-induced Liver Injury. Clin Gastroenterol Hepatol. 2016; 3565: 30309-30303.
- Colsky J, Greenspan EM, Warren TN. Hepatic fibrosis in children with acute leukemia after therapy with folic acid antagonists. AMA Arch Pathol. 1955; 59: 198-206.
- Nyfors A, Poulsen H. Liver biopsies from psoriatics related to methotrexate therapy. 1. Findings in 123 consecutive non-methotrexate treated patients. Acta Pathol Microbiol Scand A. 1976; 84: 253-261.
- Kremer JM, Lee RG, Tolman KG. Liver histology in rheumatoid arthritis patients receiving long-term methotrexate therapy. A prospective study with baseline and sequential biopsy samples. Arthritis Rheum. 1989; 32: 121-127.
- West SG. Methotrexate hepatotoxicity. Rheum Dis Clin North Am. 1997; 23: 883-915.
- Visser K, Katchamart W, Loza E, Martinez-Lopez JA, Salliot C, Trudeau J, et al. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann Rheum Dis. 2009; 68: 1086-1093.
- Roenigk HH, Auerbach R, Maibach HI, Weinstein GD. Methotrexate in psoriasis: revised guidelines. J Am Acad Dermatol. 1988; 19: 145-156.
- Curtis JR, Beukelman T, Onofrei A, Cassell S, Greenberg JD, Kavanaugh A, et al. Elevated liver enzyme tests among patients with rheumatoid arthritis or psoriatic arthritis treated with methotrexate and/or leflunomide. Ann Rheum Dis. 2010; 69: 43-47.
- Sakthiswary R, Chan GY, Koh ET, Leong KP, Thong BY. Methotrexateassociated nonalcoholic fatty liver disease with transaminitis in rheumatoid arthritis. ScientificWorldJournal. 2014; 2014: 823763.
- Zachariae H, Kragballe K, Søgaard H. Methotrexate induced liver cirrhosis. Studies including serial liver biopsies during continued treatment. Br J Dermatol. 1980; 102: 407-412.
- Laharie D, Seneschal J, Schaeverbeke T, Doutre MS, Longy-Boursier M, Pellegrin JL, et al. Assessment of liver fibrosis with transient elastography and FibroTest in patients treated with methotrexate for chronic inflammatory diseases: a case-control study. J Hepatol. 2010; 53: 1035- 1040.
- Kremer JM, Alarcón GS, Lightfoot RW, Willkens RF, Furst DE, Williams HJ, et al. Methotrexate for rheumatoid arthritis. Suggested guidelines for monitoring liver toxicity. American College of Rheumatology. Arthritis Rheum. 1994; 37: 316-328.
- Burak KW, Urbanski SJ, Swain MG. Successful treatment of refractory type 1 autoimmune hepatitis with methotrexate. J Hepatol. 1998; 29: 990-993.
- Sultan MI, Biank VF, Telega GW. Successful treatment of autoimmune hepatitis with methotrexate. J Pediatr Gastroenterol Nutr. 2011; 52: 492- 494.
- Moreno-Otero R, García-Buey L, García-Sanchez A, Trapero-Marugán M. Autoimmune hepatitis after long-term methotrexate therapy for rheumatoid arthritis. Curr Drug Saf. 2011; 6: 197-200.
- Ksouda K, Affes H, Atheymen R, Ezzeddine M, Zeghal K, Hammami S. Autoimmune hepatitis as an adverse effect of long-term methotrexate therapy. Indian J Pharmacol. 2014; 46: 649-650.
- Medina J, García-Buey L, Moreno-Otero R. Review article: immunopathogenetic and therapeutic aspects of autoimmune hepatitis. Aliment Pharmacol Ther. 2003; 17: 1-16.
- Suzuki A, Brunt EM, Kleiner DE, Miquel R, Smyrk TC, Andrade RJ, et al. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug-induced liver injury. Hepatology. 2011; 54: 931-939.
- Björnsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, et al. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010; 51: 2040-2048.
- Frenzel C, Herkel J, Lüth S, Galle PR, Schramm C, Lohse AW. Evaluation of F-actin ELISA for the diagnosis of autoimmune hepatitis. Am J Gastroenterol. 2006; 101: 2731-2736.
- Starr SP, Raines D. Cirrhosis: diagnosis, management, and prevention. Am Fam Physician. 2011; 84: 1353-1359.
- Lohse AW, Mieli-Vergani G. Autoimmune hepatitis. J Hepatol. 2011; 55: 171-182.
- DeLemos AS, Foureau DM, Jacobs C, Ahrens W, Russo MW, Bonkovsky HL. Drug-induced liver injury with autoimmune features. Semin Liver Dis. 2014; 34: 194-204.