Case Report
Brachial Artery Injury during Surgical Repair of Distal Biceps Rupture: A Report of Two Cases
Singh H*, Yang JS, Voss A, Tinsely B and Shea KP
Department of Orthopedic Surgery, University of Connecticut, USA
*Corresponding author: Hardeep Singh, Department of Orthopaedic Surgery, University of Connecticut, Health Center, 263 Farmington Ave, Farmington, CT 06034-4037, USA
Published: 29 Aug, 2016
Cite this article as: Singh H, Yang JS, Voss A, Tinsely
B, Shea KP. Brachial Artery Injury
during Surgical Repair of Distal Biceps
Rupture: A Report of Two Cases. Ann
Clin Case Rep. 2016; 1: 1110.
Abstract
Ataxia was the only presenting symptom of a large pontine melanoma secondary in a 63 year-old male after five years of follow-up. Although the brain is a common site for melanoma metastasis, brainstem involvement is uncommon and its presentation with ataxia and no cranial nerve palsies is unusual.
Keywords
Brachial artery injury; Distal biceps repair; Distal biceps rupture
Introduction
Distal biceps rupture often occurs in middle-aged men, typically as a result of a large eccentric load applied to a flexed elbow. Non-operative management of a distal biceps rupture leads to loss of approximately 20-30% flexion strength and up to 40% supination strength [1-5]. Surgical repair of a distal biceps rupture is a well-accepted treatment modality in young active patients, allowing for restoration of the anatomy of the biceps tendon and flexion and supination strength of the elbow [2,6]. Surgical repair provides excellent subjective and objective results, and early surgical repair have been advocated [3]. Significant complications of distal biceps repair have also been reported, such as lateral antebrachial nerve palsy, superficial radial nerve palsy, posterior interosseous nerve palsy, heterotopic ossification, and re-rupture. However, despite the proximity of the vascular structures, an arterial injury has never been reported in literature to our knowledge. The cases presented describe an injury to the brachial artery and highlight the complications that can arise during surgical repair of the distal biceps tendon rupture.
Case Presentation
Case 1
53 year-old male presented for evaluation of left elbow pain after two consecutive injuries to his
left elbow. The first occurred when he caught a heavy object with his left forearm and felt immediate
pain and a pop in his left elbow. The second incident occurred a week later while doing heavy lifting
and he felt a pop in his left elbow, followed by ecchymosis and pain in the left antecubital fossa.
Examination revealed full active ROM of his left shoulder, marked tenderness in the left distal biceps
area, with no palpable left distal bicep and weakness with resisted supination. MRI of the left elbow
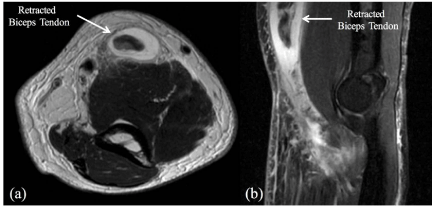
revealed a complete tear of the biceps tendon at the distal attachment site, with retraction 8 to 10cm
proximally with thickening and heterogeneous fluid surrounding the torn tendon (Figure 1).
The patient elected to have operative treatment. Fluoroscopy was used to identify the radial
tuberosity and a transverse incision was made slightly medial to avoid the lateral antebrachial
cutaneous nerve (LABCN). Marked scarring was noted in the distal biceps tendon. Scar tissue was
noted extending up into the biceps. However, despite vigorous dissection proximally, the distal
biceps tendon stump could not be identified. Profuse bleeding was noted and attempts to identify
the source of the bleeding were made. The tourniquet was deflated and a brachial artery injury was
identified. The two ends of the injured vessel were clamped for hemostasis and distal perfusion
verified with doppler ultrasound. A primary brachial artery repair was performed in concert with
vascular surgery restoring distal flow. Examination of the repair did not reveal any leakage. The
distal biceps tendon could not be identified and a biceps-to-brachialis tenodesis was performed. The
patient was observed for compartment syndrome prior to discharge. He was followed closely post-operatively and did well with minimal pain, stating his arm felt strong
but fatigues easily. He denied complaints of paresthesias and hada 2+
palpable radial pulse.
Case 2
63-year-old male presented as a transfer to our facility with
concern for limb ischemia and compartment syndrome after a distal
biceps tendon repair. The patient underwent a distal biceps tendon
repair at a stand-alone surgery center and developed swelling in the
recovery room along with an absent radial artery pulse. The patient
returned to the operating room for wound exploration at which
time an artery laceration was discovered. Due to the poor perfusion
of the patient’s hand, the patient was emergently transferred to
the main hospital. Upon arrival, he had generalized fullness of the
forearm. Pulses were intact proximally along the brachial artery
until the antecubital fossa, past which there were no palpable, or
dopplerable radial or ulnar artery pulses. CT angiogram revealed
brachial artery occlusion proximal to the elbow joint with no
distal perfusion. The patient was taken back to the operating room
in concert with the vascular team for right upper extremity limb
ischemia. A large hematoma was evacuated and the brachial artery
and its trifurcation were in the field of view. A radial artery and
an interosseous artery transection were discovered along with an
ulnar artery thrombosis, with no distal flow. An embolectomy was performed followed by primary repair of the radial artery laceration
and ligation of the interosseous artery transection. The radial artery
pulse was immediately reestablished with excellent perfusion to the
hand. Attention was turned to the dusky muscular tissue throughout
the forearm. Given these findings and the risk of reperfusion injury
it was felt most appropriate to release the volar, dorsal, and mobile
wad compartments. The flexor carpi radialis muscle was mobilized to
provide coverage of the vascular repair and negative pressure therapy
was initiated. He returned to the operating room for repeat irrigation
and debridement followed by dorsal wound closure and skin grafting
of the volar wound. He did well postoperatively with excellent
perfusion of his hand but developed partial anterior interosseous
nerve palsy with extensor lag of his ring and small finger.
Figure 1
Figure 1
(a) Axial and (b) Sagittal magnetic resonance imaging (MRI) of the
left elbow demonstrating a distal biceps tendon rupture.
Figure 2
Figure 2
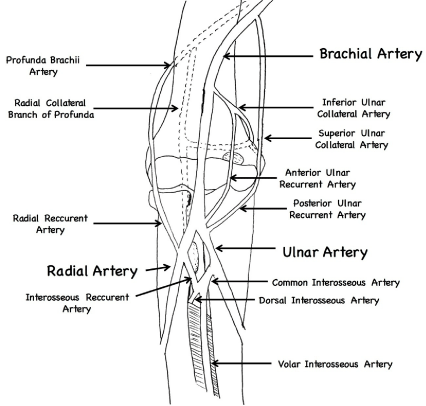
Brachial artery anatomy illustration, depicting the collateral blood
supply around the elbow.
Discussion
To our knowledge this is the first report of a brachial artery injury
during a distal biceps tendon repair. The brachial artery arises as an
extension of the axillary artery after the distal aspect of teres major
muscle and terminates at approximately one centimeter distal to the
elbow joint giving rise to the radial and ulnar arteries [7]. It is on
average 26.29 cm long and divides into its terminal branches, the radial
and ulnar arteries, at a mean of 2.99 cm distal to the intercondylar line
[7]. It runs with the median nerve during its descent to the elbow joint
and the artery is medial to the humerus proximally. It then courses
anteriorly eventually lying in between the humeral epicondyles at the
level of the elbow joint [7]. During its course, it gives off a number of different branches including the profunda brachii, nutrient artery of
the humerus, superior ulnar collateral artery, inferior ulnar collateral
artery, various muscular branches and terminates into the radial and
ulnar arteries. The anterior radial collateral, superior ulnar collateral
and inferior ulnar collateral arteries provide a rich collateral blood
flow to the distal extremity consisting of seven vessels (Figure 2)
[8,9]. Due to the close proximity of the vessels to the biceps tendon,
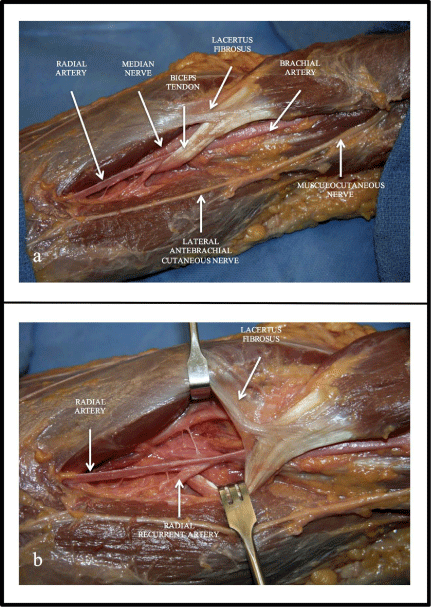
they can theoretically be injured in the surgical exposure of the radial
tuberosity for repair (Figure 3).
Bain et al. [10] used cadaveric specimens to study the distance
of the neurovascular structures to the biceps tendon. The superficial
branch of the radial nerve was on average 15mm from the biceps
tendon, posterior interosseous nerve (PIN) was on average 18mm
away, median nerve was 12mm away, radial artery was 12mm away,
and ulnar artery was 6mm away. This study highlighted the proximity
of these structures to the biceps tendon and cautions against cavalier
dissection during exposure of the radial tuberosity.
Although distal biceps repair is a relatively safe procedure to
perform, there are a number of potential complications that can arise.
The risk of encountering complications increases as the repair is
delayed and range from 8-44% [2,3,11]. Specifically, there is a higher
incidence of complications with delay greater than 4 weeks [11].
Early operative treatment allows for an easier retrieval of the biceps
tendon versus delayed treatment, where visualization of the tendon is
obscured by scar tissue [11]. Complications described in the literature
include nerve injury, persistent anterior elbow pain, heterotopic
ossification (HO), rerupture, and radioulnarsynostoses4. Injury to
the LABCN is the most frequently described nerve injury as it is
frequently encountered on the lateral aspect of the anterior incision
[3]. Injury to the LABCN can lead to a painful neuroma formation
or paresthesias in the anterolateral aspect of forearm. Although rare,
median and radial nerve palsies have been reported during surgical
exposure [3]. Rerupture of the distal biceps tendon after surgical
repair is very rare due to the protected rehabilitation programs
along with the reflex inhibition of the surrounding musculature
[12]. Heterotopic ossification has been reported in up to 10-15%
of cases and involves growth of the bone in nonosseous locations
[2,13]. Injury to the surrounding vasculature is a risk but no cases
to our knowledge have been described in the literature. Cain et al.
[11] retrospectively reviewed 198 patients with distal biceps ruptures
treated surgically and evaluated time from injury to repair, surgical
technique, and complications. There was a 36% complication rate,
which included lateral antebrachial cutaneous nerve paresthesias in
26%, radial sensory nerve paresthesias in 6%, posterior interossesous
nerve palsy in 4%, symptomatic HO in 3%, superficial infection in 2%,
and rerupture in 2% [12]. A higher complication rate was associated
with a repair performed more than 28 days after rupture [11].
Brachial artery injury can occur during exposure of the radial
tuberosity. Any profuse bleeding during radial tuberosity exposure
or proximal dissection can be a result of a vascular injury. These cases
are the first to our knowledge with a report of vascular injury during
a distal biceps tendon repair and emphasize important lessons one
should keep in mind while performing this procedure. The vessels are
in close proximity to the distal biceps tendon and the surgeon should
be careful not to perform an extensive dissection to find the tendon
stump. The dissection field should remain superficial to retrieve
the distal stump as deep dissection can injure the vessels in close
proximity (Figure 3). Following the repair and prior to closure, the
tourniquet ought to be deflated to ensure hemostasis. If hemostasis is not obtained careful surgical exploration must be performed to
identify the source of bleeding. A vascular injury was realized in case
one after deflating the tourniquet. Failure to deflate the tourniquet
prior to closure can result in a complication similar to the one
observed in case two, which further lead to the development of limb
ischemia and compartment syndrome.
The vascular injury should be clamped to provide temporary
hemostasis. Pulses in the radial artery can be palpated to ascertain
distal arterial flow and doppler ultrasound should be used to verify
distal perfusion in the radial and ulnar arteries. The proximal and
distal ends of the injured vessels need to be debrided followed by
primary arterial repair. If an immediate primary repair is unable to
be performed, the vessels can be tied off leaving long tails so that
they can be easily identified. The collateral blood flow consisting of
approximately seven vessels is able to provide adequate perfusion
distally even with an acute brachial artery transection [14]. The patient
should be transferred emergently to a tertiary care center where an
emergent vascular repair can be performed. The arterial repair should
be inspected for any leaks and distal flow in the radial and ulnar
arteries verified with Doppler ultrasound. Serial examinations of the
forearm should be performed for the development of compartment
syndrome. The surgeon should have a low threshold for performing
compartments releases as done in case two. Reperfusion injury
after vascular repair can cause further damage to the extremity
necessitating compartment releases. It is important to follow the
patient closely post operatively to monitor the patency of the vascular
repair. Appropriate anti-coagulation in concert with vascular surgery
needs to be provided to prevent thrombosis of the vascular repair.
Figure 3
Figure 3
(a) Cadaveric dissection illustrating the close proximity of the
brachial and radial artery to the distal biceps tendon. (b) Closer view of the
distal biceps tendon and its relationship to the brachial and radial artery.
Conclusion
Surgical repair of a distal biceps rupture is an acceptable method of treatment as it is a relatively safe procedure, which provides restoration of flexion and supination. Complications associated with surgical repair of the distal biceps rupture include nerve damage, anterior elbow pain, heterotopic ossification, rerupture, and radioulnar synostoses. Brachial artery injury is a rare complication of distal biceps rupture repair that should be identified quickly, clamped to control bleeding, and repaired primarily. Distal extremity perfusion should be verified using Doppler ultrasound to identify arterial flow in the radial and ulnar arteries. These cases highlight the importance of vigilant surgical dissection when performing a distal biceps repair and deflating the tourniquet prior to closure.
References
- Baker BE, Bierwagen D. Rupture of the distal tendon of the biceps brachii. Operative versus non-operative treatment. J Bone Joint Surg. 1985; 67: 414-417.
- Cohen MS. Complications of distal biceps tendon repairs. Sports med arthrosc. 2008; 16: 148-153.
- Kelly EW, Morrey BF, O'Driscoll SW. Complications of Repair of the Distal Biceps Tendon with the Modified Two-Incision Technique. J Bone Joint Sur. 2000; 82: 1575-1581.
- Meherin JM, Kilgore ES. The treatment of ruptures of the distal biceps brachii tendon. Am J Surg. 1960; 99: 636-640.
- Norman WH. Repair of avulsion of insertion of biceps brachii tendon. Clin orthop relat res.1985; 193: 189-194.
- Bisson L, Moyer M, Lanighan K, Marzo J. Complications associated with repair of a distal biceps rupture using the modified two-incision technique. J Shoulder Elbow Surg. 2008; 17: 67S-71S.
- Patnaik V, Kalsey G, Singla RK. Branching pattern of brachial artery-A morphological study. J Anat Soc India. 2002; 51: 176-186.
- Marcheix B, Chaufour X, Ayel J, Hollington L, Mansat P, Barret A et al. Transection of the brachial artery after closed posterior elbow dislocation. J Vasc Surg. 2005; 42: 1230-1232.
- Thompson JC, Netter FH. Netter's Concise Atlas of Orthopaedic Anatomy: Icon Learning Systems; 2002.
- Bain GI, Prem H, Heptinstall RJ, Verhellen R, Paix D. Repair of distal biceps tendon rupture: A new technique using the endobutton. J Shoulder Elbow Surg. 2000; 9: 120-126.
- Cain RA, Nydick JA, Stein MI, Williams BD, Polikandriotis JA, Hess AV. Complications Following Distal Biceps Repair. J Hand Surg. 2012; 37: 2112-2117.
- Katolik LI, Fernandez J, Cohen MS. Acute failure of distal biceps reconstruction: a case report. J Shoulder Elbow Surg. 2007; 16: e10-e12.
- Karunakar MA, Cha P, Stern PJ. Distal Biceps Ruptures: A Followup of Boyd and Anderson Repair. Clinical orthopaedics and related research. 1999; 363: 100-107.
- Menzoian JO, Corson JD, Bush HL, LoGerfo FW. Management of the upper extremity with absent pulses after cardiac catheterization. A Jour Surg. 1978; 135: 484-487.