Case Report
A 32-Year-Old Woman with Intestinal Bleeding
Piero Tartaro1* and Tiffany Chan2
1Division of Gastroenterology, Sunnybrook Health Sciences Centre, Canada
2Department of Medicine, University of Toronto, Canada
*Corresponding author: Piero Tartaro, Division of Gastroenterology, Sunnybrook Health Sciences Centre, Room HG64, 2075 Bayview Avenue, Toronto, Ontario M4N 3M5, Canada
Published: 27 Aug, 2016
Cite this article as: Tartaro P, Chan T. A 32-Year-Old
Woman with Intestinal Bleeding. Ann
Clin Case Rep. 2016; 1: 1106.
Abstract
We present a case report of a patient from the Philippines who presented with acute onset lower gastrointestinal bleeding, diagnosed with intestinal tuberculosis. This case highlights one of the atypical presentations of extra-pulmonary tuberculosis. Diagnosis requires endoscopic evaluation not only for histopathology but tissue culture, in order to distinguish this from other mimickers like inflammatory bowel conditions. Although more common in countries known to have a high burden of disease, including the Philippines, abdominal intestinal tuberculosis should be considered in the differential diagnosis of patients presenting with vague abdominal pain, rectal bleeding, and caseating granulomas on histopathology.
Keywords
Mycobacterium tuberculosis; Intestinal tuberculosis; Lower gastrointestinal bleed
Case Presentation
A 32 year-old previously healthy female presented to the emergency department (ED) with a
two-day history of hematochezia. She took no medications and had immigrated to Canada from
the Philippines 8 years prior. She initially noticed bright red blood mixed with normal bowel
movements, followed by loose bloody stools. On the background of this was a two-month history
of intermittent right lower quadrant abdominal pain, associated with unintentional weight loss but
no fevers or night sweats. There was no recent travel history or antibiotic use. She denied extraintestinal
manifestations of inflammatory bowel disease, including uveitis, aphthous stomatitis,
rashes, or arthritis.
On presentation, she was hemodynamically stable and afebrile. Physical examination
revealed mild right lower quadrant abdominal tenderness with deep palpation, but was otherwise
unremarkable. Initial laboratory investigations demonstrated a microcytic anemia (hemoglobin
level 99g/L, MCV 77.6), normal white blood cell count (7.5x109/L) and platelets (166 x109/L), normal
inflammatory markers (erythrocyte sedimentation rate 16mm/h and C-reactive protein 1mg/L),
and normal liver profile (aspartate transaminase 15U/L, alanine transaminase 8U/L, and alkaline
phosphatase 36U/L). Of note, she had presented to a different ED the day prior and her hemoglobin
was noted to be 116g/L. Abdominal X-ray was normal. Given the drop in hemoglobin, the patient
was admitted to hospital for further investigations, including a colonoscopy, by the Gastrointestinal
(GI) team.
Diagnosis
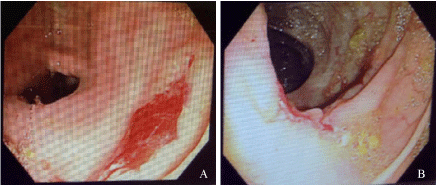
Colonoscopy revealed discrete ulcers in the cecum and ascending colon, without intervening
mucosal changes (Figure 1). There was no evidence of active bleeding and only one 8mm ulcer in
the ascending colon had a small blood clot within in. The terminal ileum and remaining segments of
colon were endoscopically normal (Figure 2). Multiple biopsies were taken and the histopathology
of the ulcer edges revealed mild to moderate chronic colitis with necrotizing granulomas. The patient
had no recurrence of lower GI bleeding (LGIB) during her hospitalization and was subsequently
discharged home with outpatient GI follow-up.
The colonic ulcer biopsies were negative for acid fast bacilli (AFB) on smear microscopy,
but subsequent tissue cultures were positive for Mycobacterium tuberculosis (TB) complex. The
patient was referred to an infectious disease specialist and empirically started on rifampin (RMP)
600mg daily, isoniazid (INH) 300mg daily, pyrazinamide (PZA) 900mg daily, ethambutol (EMB)
675mg daily, and pyridoxine (vitamin B6) 50mg daily. Additional investigations, including three
consecutive expectorated sputum samples, chest X-ray, and HIV testing, were all negative. On
further questioning, it was discovered that her father had been previously treated for TB and she in
fact had a positive Mantoux skin test in 2014 that was not further investigated or treated.
Final sensitivities revealed a fully susceptible organism. The
ethambutol was discontinued and the patient completed a two-month
course of rifampin, isoniazid and pyrazinamide, following which she
began a four month course of rifampin and isoniazid. Clinically, she
remained well with no further episodes of LGIB and had no adverse
reactions to her medications.
Figure 1
Figure 2
Discussion
Tuberculosis (TB) is a multi-systemic bacterial infection caused
by Mycobacterium tuberculosis and affects approximately onethird
of the world’s population. In 2013, 9 million individuals were
infected with TB worldwide, most of whom had lived in one of the 22
countries (including the Philippines) known to have a high burden of
disease [1]. According to the WHO 2015 global TB report, there were
243,379 new cases of TB, of which 4,161 were extra-pulmonary cases
in the Philippines [2]. Furthermore, there were 108 HIV-positive TB
patients in 2014, a nearly 4-fold increase compared to 2013. The rise
in prevalence of HIV-positive patients may be a contributing factor
in the resurgence of TB across these nations. The correlation between
HIV and TB infection is well recognized in developed countries, with
TB becoming an index disease to screen for HIV, thereby identifying
undiagnosed HIV patients and initiating early anti-retroviral therapy
as needed.
While pulmonary TB is the most common manifestation of
disease, extra-pulmonary TB may occur as a part of primary or
reactivated infection. Abdominal TB is the sixth most common
type of extra-pulmonary TB after lymphatic, genitourinary, bone
and joint, miliary, and meningeal involvement. It may be acquired
through hematogenous spread, direct invasion from adjacent organs,
swallowing infected sputum, or ingesting contaminated food [3].
It subsequently infects multiple organs including the GI tract,
peritoneum, mesenteric lymph nodes, liver, spleen, and pancreas.
In a recent retrospective review article that identified 57 patients
with abdominal TB in a developed country, the most common area of involvement was the GI tract and 66% of patients had evidence
of bowel thickening on their CT scan [4]. Although our patient did
not undergo abdominal CT scan, her primarily site of involvement
on endoscopy was her GI tract. Intestinal lesions have been shown
to occur in the ileocecal region in up to 75% of cases, thought to
be secondary to the area’s minimal digestive activity and increased
physiological stasis, higher fluid and electrolyte absorption, and
greater lymphoid tissue [3].
In our patient, her chief complaint of hematochezia would have
initially favoured a diagnosis of hemorrhoids, which is the most
common cause of rectal bleeding in patients under the age of 50 [5].
However, significant lower GI bleeding resulting in an acute drop
in hemoglobin is uncommon with hemorrhoids, and usually causes
bright red blood coating the stool rather than being mixed in with
stool as seen in our patient. Clinical manifestations of intestinal TB are
non-specific, including abdominal pain, anorexia, nausea, vomiting,
weight loss, fever, intestinal obstruction, perforation peritonitis, and
ascites [3]. It is a common mimicker of other inflammatory bowel
conditions, particularly Crohn’s disease (CD), given the similarities
in clinical presentation and pathology [6]. Our patient’s age, female
gender, and chronic right lower quadrant pain associated with weight
loss would have made CD a more likely diagnosis over intestinal
TB, especially given that it is more commonly encountered in North
America. Furthermore, patients with CD can present with sudden
and brisk hematochezia when an intestinal ulcer erodes into an
artery, whereas intestinal TB presenting with LGIB is rare. A 2009
literature review found only 10 cases of intestinal TB presenting with
LGIB [7], most often from the ileocecal region.
Both diseases are characterized by chronic granulomatous
inflammation, but Crohn’s disease is typically non-caseating while
intestinal TB is caseating [6,8], the latter of which we saw on our
patient’s pathology. Ultimately, distinguishing between these two
diseases is essential for management. Unnecessary anti-TB drugs
could cause toxicity and delays treatment of the actual disease, while
incorrect treatment with steroids and immunosuppressive agents
could cause fatal dissemination of TB [6].
The diagnosis of intestinal TB is most often made after endoscopic
evaluation and biopsy of the affected area. While our patient had
evidence of right-sided colonic ulcers, she did not have other typical
features of intestinal TB including inflammatory strictures or
hypertrophic lesions [6]. Histopathologies of the involved areas, as
described above, often shows non-specific findings and are present in
less than one-third of intestinal TB cases [6]. Smear microscopy with
auramine staining is the most widely used test for detection of AFB
[9]. Its limitations lie in its variable sensitivity depending on the type
of specimen, patient population, and experience of the microscopist
[10]. Furthermore, the presence of AFB in biopsies has only been
reported to be positive in 5-11% of intestinal TB cases [8,11]. This is
consistent with our patient’s findings of a negative AFB smear and
positive TB culture. Thus, mycobacterial culture is the gold standard
in making a diagnosis but may take up to 8 weeks, especially if there
is a low burden of disease [10]. Ultimately, difficulties in clinical
diagnosis, lack of specific biologic markers, and long incubation
time for cultures often leads to a delay in diagnosis and definitive
treatment.
Treatment of intestinal tuberculosis depends on the clinical
presentation and associated complications. Patients who develop
bowel obstruction, perforation, intestinal ischemia, or severe
bleeding often require urgent exploratory laparotomy, followed by
post-operative anti-tubercular drugs [7]. Otherwise, management
is predominately medical. A large prospective cohort study in India
found that follow-up colonoscopy to document endoscopic resolution
was unnecessary, as it did not provide any additional changes to
management in those patients who successfully completed treatment
and remained asymptomatic [9].
Conclusion
We presented a case report of intestinal tuberculosis in a previously healthy woman from the Philippines with hematochezia. Her clinical presentation, although non-specific, was atypical for intestinal tuberculosis given the lower GI bleeding. Endoscopic evaluation with biopsy and tissue culture was ultimately required for definitive diagnosis. Abdominal intestinal tuberculosis, although uncommon, should be considered in the differential diagnosis of patients presenting with vague abdominal pain and rectal bleeding, with caseating granulomas on histopathology.
References
- Global Tuberculosis (TB). Centers for Disease Control and Prevention. 2014.
- Global tuberculosis report 2015. World Health Organization 2015.
- Marshall JB. Tuberculosis of the gastrointestinal tract and peritoneum. Am J Gastroenterol. 1993; 88: 989-999.
- Tan KK, Chen K, Sim R. The spectrum of abdominal tuberculosis in a developed country: a single institution’s experience over 7 years. J Gastrointest Surg. 2009; 13: 142-147.
- Korkis AM, McDougall CJ. Rectal bleeding in patients less than 50 years of age. Dig Dis Sci. 1995; 40: 1520-1523.
- Sood A, Midha V, & Singh A. Differential Diagnosis of Crohn’s Disease Versus Ileal Tuberculosis. Curr Gastroenterol Rep. 2014; 16: 418.
- Kela M, Agrawal A, Sharma R, Agarwal R, Agarwal V. Ileal tuberculosis presenting as a case of massive rectal bleeding. Clin Exp Gastroenterol. 2009; 2: 129-131.
- Awasthi S, Saxena M, Ahmad F, Kumar A, Dutta S. Abdominal Tuberculosis: A Diagnostic Dilemma. J Clin Diagn Res. 2015; 9: ECO1-ECO3.
- Mukewar S, Mukewar S, Ravi R, Prasad A, Dua KS. Colon Tuberculosis: Endoscopic Features and Prospective Endoscopic Follow-Up After Anti- Tuberculosis Treatment. Clin Transl Gastroenterol. 2012; 3: e24.
- Canadian Tuberculosis Standards, 7th Edition. Canadian Thoracic Society. 2013.
- Alvares JF, Devarbhavi H, Makhija R, Rao S, Kottoor R. Clinical, colonoscopic, and histological profile of colonic tuberculosis in a tertiary hospital. Endoscopy. 2005; 37: 351-356.