Case Report
Transthoracic Thermodilution Measurements Guiding Management of Postoperative Cardiogenic Shock Case Report
Clark CA1*, Teegarden BMT2, Kottkamp GS1, Field LC1 and Rieke H1
1Department of Anesthesia and Perioperative Medicine, Medical University of South Carolina, USA
2Department of Anesthesia, University of Texas Branch, USA
*Corresponding author: Carlee A Clark, Department of Anesthesia and Perioperative Medicine, Medical University of South Carolina
Published: 25 Aug, 2016
Cite this article as: Clark CA, Teegarden BMT, Kottkamp
GS, Field LC, Rieke H. Transthoracic
Thermodilution Measurements
Guiding Management of Postoperative
Cardiogenic Shock Case Report. Ann
Clin Case Rep. 2016; 1: 1102.
Abstract
This case report describes the complicated postoperative course of a 73 year-old male patient with pre-existing congestive heart failure after uneventful hepaticojejunostomy for benign biliary stricture. The hemodynamic and respiratory failure resulting from cardiogenic shock was successfully treated by using transthoracic thermodilution measurements to guide the choice of catecholamines, antiarrhythmic drugs, and repeated attempts at cardioversion. Determination of global end-diastolic volume, extravascular lung water, thermodilution-calibrated pulse-contour derived continuous cardiac output as well as the ratio of cardiac output per global end-diastolic volume (cardiac function index) was deemed to be advantageous for management of this clinically challenging patient.
Keywords
Global end diastolic volume (GEDV); Extravascular lung water index (ELWI); Cardiac function index (CFI); Pulmonary artery catheters (PAC); Ejection fraction (EF)
Introduction
Standard recommendations for assessment of hemodynamic instability, particularly in sepsis,
are often limited to heart rate and blood pressure together with a clinical holistic impression [1]. Further details obtained by ultrasound evaluation, chest x-rays, imaging studies and other various
invasive methods are often based on individual preferences, leading to more or less heuristic
therapeutic concepts [2].
Currently,use of stroke volume variation for estimates of the volume status of a mechanically
ventilated patient seems to be widely used, which also includes estimates of cardiac output from
arterial blood pressure curves [3]. Availability of this newer technology and concerns regarding the
inaccuracy of assessments of the intravascular volume status from central venous pressure and the
pulmonary capillary wedge pressure during positive pressure ventilation have led to reduced usage
of pulmonary artery catheters (PAC) [4].
Ultrasound parameters have been introduced and become major tools because of their
excellent support of clinical impressions, showing moving pictures of anatomical structures that are
meaningful for hemodynamic function [5]. However, ultrasound imaging is often hard to obtain
due to postsurgical wounds and dressings, posture and general condition of the patient. Moreover,
even trans-esophageal echocardiographycannot offer the continuity of data for an extended time
course secondary to patient sedation requirements.
The transthoracic thermodilution technique with volumetrically determined global end diastolic
volume (GEDV) has been suggested in recent decades [6]. It allows consideration of the ratio of
cardiac output to GEDV, which is termed “cardiac function index” (CFI) [7]. In fact, the CFI is
physiologically similar to the classic ejection fraction (EF), representing principally the amount of
blood being ejected from the heart per pre-ejection filling [7].
Beyond that, the determination of extravascular lung water index (ELWI) allows for estimating
the leakage of water from the intravascular space into lung tissue. For conditions with high intrathoracic
blood volume (GEDI provides a measure of this), elevated ELWI indicates pulmonary
edema from congestion, versus high ELWI with reduced intra-thoracic blood volume. This clinical
situation is typical for capillary leak secondary to endothelial dysfunction and inflammation [8].
In recent years, most reports have focused on the use of the
transthoracic thermodilution for hemodynamic management of
sepsis, where detailed information helps guide controlled volume
resuscitation and management of impaired cardiac function [3].
This case report attempts to describe the advantages of basing
clinical management of a patient with severe cardiac failure after
abdominal surgery on the therapeutic concept derived from data
obtained from transthoracic thermodilution.
Case Presentation
The patient, a well optimized 73 year-old male with a medical
history of ischemic cardiomyopathy and paroxysmal atrial
fibrillation, underwent an uneventful hepaticojejunostomy for
recurrent choledocholithiasis. On the morning of postoperative day
one, the (patient developed respiratory failure, altered mental status
and atrial fibrillation with rapid ventricular rate requiring admission
to the intensive care unit (ICU) (Figure 1). Upon admission to the
ICU he was hypoxemic (SaO2p ~80%) and tachycardic (150–160/
min) despite attempts to slow his rapid ventricular rate (RVR) with
metoprolol and diltiazem.
Regarding his worsening mental status and clinical picture,
endotracheal intubation and positive pressure ventilation was
immediately initiated under sedation with fentanyl and propofol. A
central line was placed into the right internal jugular vein, as well as a 20 gauge thermistor arterial catheter (PiCCO Pulsion®) into the right
femoral artery. Norepinephrine was started for maintenance of mean
arterial blood pressure (MAP) > 60 mmHg. Initial chest x-ray showed
right lower lobe consolidation, possibly indicative of aspiration as
well as mild interstitial edema (Figure 2). Broad-spectrum antibiotic
coverage with vancomycin and cefepime was started for possible
sepsis. Anticoagulation with a heparin drip was initiated for the
concern for an underlying pulmonary embolism. A transthoracic
ultrasound study showed global hypokinesis with an estimated
ejection fraction < 20%, but could not comment on the volume
status. Visualization of the inferior vena cava was not attempted due
to surgical wound dressings. Initial labs showed a troponin of 13.15
ng/ml, arterial lactate of 3.80 mmol/L and procalcitonin of 14.08
microgram/l.
The transthoracic thermodilution measurement revealed
significantly reduced cardiac index of 1.6 [l/min*m2], global enddiastolic
volume index within the upper range of normal values
with slightly elevated extravascular lung water, thus indicating mild
cardiogenic pulmonary edema. Systemic vascular resistance was
close to the upper range of normal values. Stroke volume was severely
reduced due to supraventricular tachyarrhythmia.
Cardiology suggested continued titration of inotropes and
vasopressors to an appropriate cardiac index. An attempt to improve
cardiac output with dobutamine resulted expectedly in worsened
tachyarrhythmia. Infusions of epinephrine (4 – 6 micrograms /
min) and norepinephrine (6 – 10 micrograms / min) were initiated
which transiently stabilized the MAP, but this neither improved
cardiac index nor cardiac function index. On postoperative day two,
the cardiac index and stroke volume remained below normal, so
when the patient’s heart rate increased acutely to 163 bpm, electrical
cardioversion was attempted. Initial failure to convert to sinus
rhythm resulted in electrolyte optimization and the initiation of an
amiodarone infusion (Figure 3). Repeat cardioversion was successful
and the resulting sinus rhythm significantly improved stroke volume
(Table 1).
Despite the concern for sepsis and its need for increased preload,
the patient remained afebrile with negative cultures. Controlled
preload reduction was begun while continuously measuring GEDI
and CFI and MAP support with norepinephrine and vasopressin.
Although the GEDI was found to be within the normal range at
the beginning of this attempt, the cardiac function index improved
slightly after a 20 mg dose of Furosemide. Reduction of GEDI from 702 ml to 609 ml was achieved within the next few hours. At this time the cardiac function index was found to be plateauing greater than
3 and was considered to be the best possible result under the given
circumstance.
The hemodynamic function continued to improve throughout
postoperative day two, as noted by a down-trending troponin level
from 17.04 ng/mL to 7.29 ng/mL, lactate decrease to 1.13 mmol/L and
decreasing need for catecholamine administration. The patient was
extubated on postoperative day three; a transthoracic echocardiogram
on postoperative day seven showed a significantly improved EF of
nearly 50%. The patient was transferred out of the ICU and eventually
discharged to home.
Discussion
This patient was clinically challenging because his hemodynamic
failure could have been interpreted as postoperative systemic
inflammation response, sepsis, an exacerbation of congestive heart
failure or even a pulmonary embolus. Assuming one of these
diagnoses , the underlying pathophysiology would have required
different strategies of fluid management and pharmacologic
approaches than the one that ultimately led to his improvement.
“Blind” attempts based on standard hemodynamic monitoring and
the clinical holistic impression of how the patient presented could
have caused a prolonged phase of “experimental” treatment, which
might have contributed to worsened myocardial ischemia and
potential fatal outcome [9].
Transthoracic thermodilution technology has a long history that
has been extensively described elsewhere, and can be considered
clinically proven [7]. Its advantage could be attributed to a real
semi-continuous volumetric measurement of preload as opposed
to other practical assessments of the volume status [6]. All in all, this technology offers independence from cardiac arrhythmia, as
opposed to stroke volume or pulse pressure variation measurements.
Although the continuous cardiac output is based on calculations of
stroke volume derived from the area under the curve of the intraarterial
blood pressure measurement, and with this one has to assume
reduced accuracy during arrhythmia, the results are internally
correlated to the thermodilution derived cardiac output and stroke
volume. Hence, there is a “calibration” of the continuous cardiac
output.
Sepsis usually requires initial aggressive fluid resuscitation that can
be guided by measuring extravascular lung water. This is particularly
helpful, because the inflammatory response leads to capillary leak and
extravasation into the lung tissue [8]. In our case there was only a
slightly elevated ELWI with an elevated intra-thoracic blood volume,
indicating a more likely cardiogenic pulmonary edema.
Visualization of the inferior vena cava could be impeded by by
abdominal wound and dressing, as it was in this case, but there is also
the limitation of easily deriving continuous numeric values like cardiac
output, systemic vascular resistance and values indicative of preload.
The ultrasound imaging on the other hand offers the unbeatable
advantage of showing real-time movement and dimensions of the
various anatomical structures of the heart and the great vessels. For
the experienced eye there might be nothing comparable to this, even
if there would be an underlying pathologic cardiac anatomy [5].
Finally the pulmonary artery catheter would have had the risk of
iatrogenic arrhythmia on top of the already existing tachyarrhythmia.
Beyond that there is the unreliable information about volume with
regards to preload derived from PCWP and CVP [10]. The remaining
advantage of the PAC would lie in the possibility of getting mixed
venous oxygen saturation to calculate oxygen balance, although the
SVO2 from the central line may be a useful alternative.
In conclusion this report endorses using transthoracic
thermodilution technology for clinically challenging cases in the
setting of perioperative and critical care medicine.
Figure 1
Figure 2
Figure 2
Chest X-ray with right lower lobe consolidation, possible aspiration as well as mild interstitial edema.
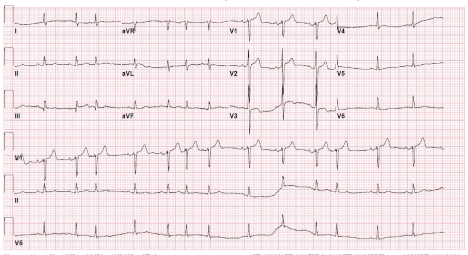
Figure 3
Figure 3
12 lead ECG following third cardioversion after potassium, magnesium and amiodarone administration.
Figure 4
Table 1
References
- Lassman AB, De Angelis LM. Brain metastases. Neurol Clin N A. 2003; 21: 1-23.
- Posner JB, Chernik NL. Intracranial metastases from systemic cancer. Adv Neuro. 1978; 19: 579- 592.
- Hunter KMF, Rewcastle NB. Metastatic neoplasms of the brainstem. Can Med Ass J. 1968; 98: 1- 7.
- Ginaldi S, Wallace S, Shalen P, Luna M, Handel S. Cranial computed tomography of malignant melanoma. AJR Am J Roentgenol. 1981; 136: 145-149.