Case Report
Fresh Frozen Homologous Bone Graft: An Atrophic Maxilla Reconstruction. A Case Report
Maiorana C1*, Pieroni S2, Roncucci R2, Poli PP2 and Beretta M2
1Oral Surgery, Fondazione IRCCS Cà Granda – Ospedale Maggiore Policlinico, Italy
2Maxillofacial Surgery and Dentistry Unit, Fondazione IRCCS Cà Granda – Ospedale Maggiore Policlinico, Italy
*Corresponding author: Carlo Maiorana, Maxillofacial Surgery and Dentistry Unit, Fondazione IRCCS Cà Granda – Ospedale Maggiore Policlinico, Via Commenda 10, 20122, Milan, Italy
Published: 09 Aug, 2016
Cite this article as: Maiorana C, Pieroni S, Roncucci R,
Poli PP, Beretta M. Fresh Frozen
Homologous Bone Graft: An Atrophic
Maxilla Reconstruction. A Case Report.
Ann Clin Case Rep. 2016; 1: 1077.
Abstract
Background: Reconstruction of severely resorbed jaws requires different surgical procedures to
restore the correct bone morphology. These procedures often involve the use of bone substitutes
or the harvesting of autologous bone from a donor site. Autologous bone is still regarded as the
“gold standard” for augmentation procedures because of its osteogenic potential, but this graft
has limited availability and the surgical harvesting procedures can cause additional morbidity to
the patient. Differently from heterologous bone or alloplatics substitutes, homologous bone has
intrinsic osteoinduction properties in addition to osteoconductivity. Despite these benefits, the
documented use of homologous block allografts in the treatment of alveolar ridge atrophy is limited
in the literature. The purpose of this case report is to evaluate the suitability of deep-frozen allograft
for ridge augmentation procedures in severely atrophic maxillae and to analyze the clinical success
of dental implants inserted after grafting and prosthetic rehabilitation.
Methods: A 50-years-old patient in general good health with a total superior edentulism and
presenting a severe athrophic maxilla has been subjected, under general anesthesia, to ridge
reconstruction with appositional homologous fresh frozen bone (FFB) block grafts pre-contoured
onto a stereolithographic model. After 8 months, 6 rough-surface implants were placed and the
fixation screws were removed. Implants were left unloaded while a total provisional prosthesis was
delivered to the patient. After 6 months a new surgery was scheduled to obtain implant exposure
and finally, 5 months later, the definitive overdenture prosthesis was completed.
Results: A radiological and clinical follow-up evaluation was conducted once a year. After 4 years
the patient is still in good condition and no complications were shown.
Conclusion: The use of homologous FFB grafts present some advantages compared to other
biomaterials such as osteoconductive and osteoinductive qualities, reduction of postoperative
discomfort for the patient, graft availability and reduction of operating time. The author’s experience
support the hypothesis that fresh-frozen bone allografts can be successful as graft material for the
treatment of important maxillary ridge defects.
Keywords: Fresh frozen bone; Homologous block allografts; Atrophic maxillae
Introduction
Reliable rehabilitation of the alveolar ridge with endosseous implants requires sufficient quality and quantity of the alveolar bone to achieve both implant stability and a successful esthetic outcome of the rehabilitation. Bone resorption after tooth loss is usually progressive and irreversible and is more prominent in the first year [1,2]. Resorption can be either vertical or horizontal, or both. It contributes to the reduction of bone volume and an unfavorable maxilla-mandibular relation [3]. Reconstruction of severely resorbed jaws requires different surgical procedures depending on the severity of the bone atrophy. These procedures often involve the use of bone substitutes or the harvesting of autologous bone from a donor site. Autologous bone is believed to be the most effective bone graft material, and is still regarded as the “gold standard” for augmentation procedures because of its osteogenic potential, but this graft has limited availability and the surgical harvesting procedures can cause additional morbidity to the patient [4]. Biomaterials alternative to autologous bone are alloplastic substitutes (such as hydroxyapatite and calcium carbonate) and allogeneic substitutes (such as demineralized freeze-dried bone allograft and heterologous bone from bovine, swin or equine) [5]. Heterologous bone can be used either alone or in combination with autologous bone and has fundamental characteristics of biocompatibility and osteoconductivity and produces a good scaffold for new bone formation. It is widely used for vertical and horizontal augmentation, sinus lift procedure and socket preservation. Differently from heterologous bone substitutes, homologous bone has intrinsic osteoinduction properties in addition to osteoconductivity. Osteoinduction is the process by which mesenchymal stem cells from a host are recruited to differentiate into chondroblasts and osteoblasts, which, in turn, form new bone through the process of endosteal ossification. This specialized process is moderated primarily by growth factors, such as bone morphogenetic proteins (BMP) -2, -4 and -7, platelet derived growth factor, interleukins, fibroblast growth factors, granulocyte-macrophage colony-stimulating factors, and angiogenic factors such as vascular endothelial growth factor [6]. Allogenic bone grafts, including fresh-frozen bone, are all harvested from cadaveric sources, and are successively processed and stored in different ways [7]. Despite these benefits, the documented use of block allografts in the treatment of alveolar ridge atrophy is limited in the literature. A concern with bone allograft is its antigenicity. However, histological and immune-response assessments showed no signs of antigenic reaction to the use of fresh-frozen allograft in the treatment of large bone defects [8]. The purpose of this case report is to evaluate the suitability of deep-frozen allograft for ridge augmentation procedures in severely atrophic maxillae and to analyze the clinical success of dental implants inserted after grafting and prosthetic rehabilitation.
Case Report
A 50-years-old patient presented at the Center for Edentulism and Jaw Atrophies (Maxillofacial Surgery and Dentistry Unit. Fondazione IRCCS Cà Granda – Ospedale Maggiore Policlinico) with a total edentulism in presence of an atrophic maxilla (class V according to Cawood & Howell atrophy classification) (Figure 1 and 2). The patient was in a good general health with a good compliance, however allergy to salicilate and a compensed ipertension were referred. On the basis of clinical evidence has been offered to the patient , in order to avoid autologous bone withdrawal, to undergo a surgical reconstruction with omologous fresh frozen bone graft (FFB). During the visit, the patient was informed about the proposed elective FFB grafting procedure. The pre-operative routine included blood tests, the signature of the informed consent form for the surgery and of a specific informed consent form for the use of the bone graft from the regional Musculoskeletal Tissue Bank ([MTB] - Gaetano Pini Orthopaedic Institute, Milan, Italy). MTB supplied the informed consent forms for the FFB grafting procedure, together with the application forms for the bone specimen (including information on its origin, shape and size). Once the specimens have been harvested, they were examinated by the MTB together with blood samples to be subjected to serologic tests. The processing of fresh allogenic bone consisted in an initial disinfection with a polychemotherapeutic solution (for 72 hours at -4°C), saline lavage, and the cutting into blocks which were then packed in double sterile bags and stored in tanks at a constant temperature of – 80°C.
Preoperative reconstructive procedure
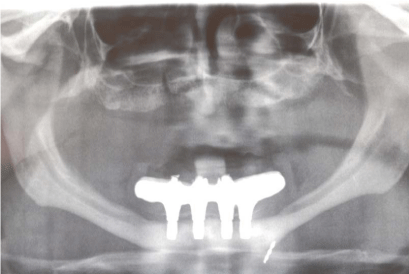
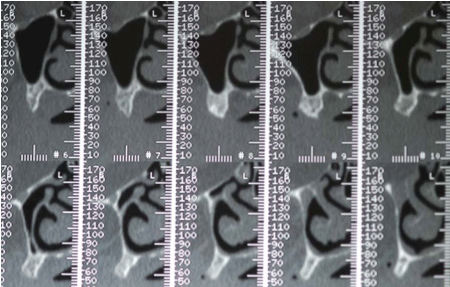
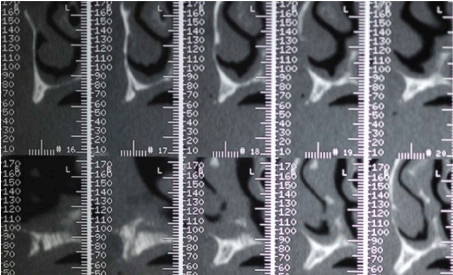
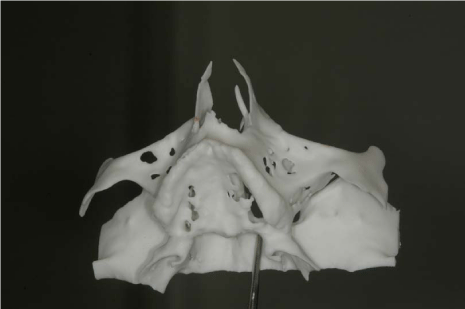
Before surgery, the patient received proper oral hygiene instructions and a professional oral hygiene treatment. Patient was also asked to make rinses with chlorhexidine 0,2% (Dentosan®, Recordati S.p.A., Milan, Italy) three times a day starting from one week before the surgery up to the sutures removal. The antibiotic therapy consisted of 2 g of amoxicillin clavulanate (Augmentin®, GlaxoSmithKline S.p.A., Verona, Italy) 1 hour before the surgery and 1 g twice daily for 7 days starting 1 hour after the surgery. The patient underwent pre-operative radiological examinations, including orthopantomographs, a computed tomography (CT) and a cephalometric lateral side (Figure 3-5). Since the patient presented a skeletal class III, it was decided to graft the FFB onlay in a J-Shaped veneer position in order to obtain enough ridge volume to allow implants positioning and to correct the intermaxillary relationship. Finally, a stereolitographic model was also required (Figure 6).
Surgical reconstructive procedures
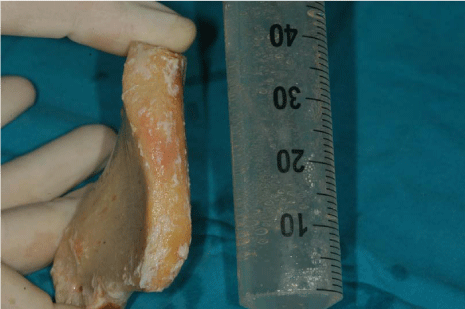
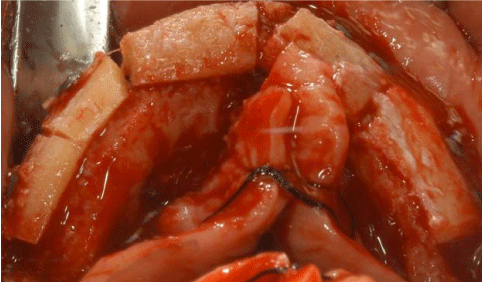
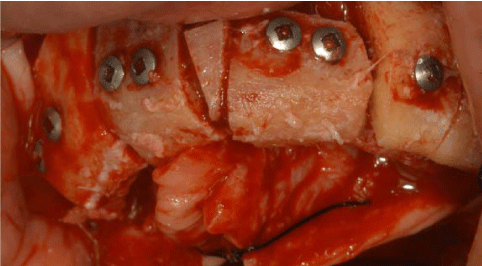
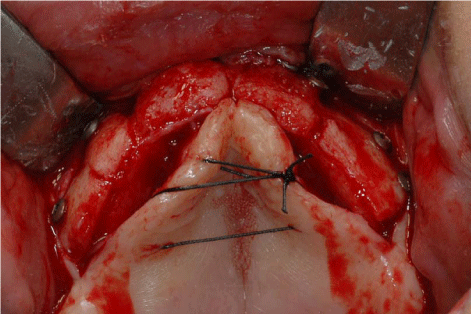
The surgical procedures were performed under general anaesthesia. On the day of surgery, a sealed container with the graft preserved under controlled temperature was delivered. It was then transferred to the operating room, where the double bag was opened in a sterile environment and the tissue specimen was defrozen in an abundant solution of saline and rifampicin at a temperature of 37° for one hour, in compliance with the instructions provided by the reference MTB. After defreezing it, it was debrided if necessary to remove non-bony tissues, cut into blocks and contoured or morcellized, based on the treatment plan (Figure 7). Before the incision, 8 mg of dexamethasone phosphate (Soldesam®, Laboratorio Farmacologico Milanese s.r.l., Varese, Italy) were intravenously injected to reduce the post-operative swelling. Local ischemia was induced by infiltration with articaine/epinephrine 1:100.000. A full thickness flap was elevated from 1.7 to 2.7 to expose the bone defects and a bilateral sinus lift with heterologous bone graft (Bioss®, Geistlich Pharma AG, Wolhusen, Switzerland). Allogenic fresh-frozen cortico-cancellous block grafts pre-contoured onto a stereolithographic model, were used to replace the missing bone. Before fixing the homologous onlay blocks with osteosynthesis screws, cortical perforations of the recipient bed were performed with a 1.5 mm diameter carbide bur) (Figure 8 and 9). The bone grafts were then covered with heterologous bone graft (Bioss®, Geistlich Pharma AG, Wolhusen, Switzerland), which was maintained in situ by means of resorbable collagen membranes (Biogide®, Geistlich Pharma AG, Wolhusen, Switzerland). The surgical wound was then sealed with horizontal mattress sutures and interrupted sutures, after releasing the flap by means of periosteal incisions.
Postoperative procedures
The forms supplied by the MTB were filled in and sent back to the MTB within a few days following surgery. The control of the patient post-operative pain was obtained by oral administration of 500 mg of paracetamol and 30 mg of Ketorolac (Lixidol®, Hoffman-La Roche, Basilea). The patient was instructed to use a chlorhexidine 0.2% (Dentosan®, Recordati S.p.A., Milan, Italy) rinse twice daily until suture removal. Sutures were removed after 14 days. Patients were placed on a soft diet during the first 3 months after surgery to limit occlusal loading and reduce micromovements, which might interfere with osseointegration. Health conditions were monthly checked, up to the second surgical step.
Implant placement
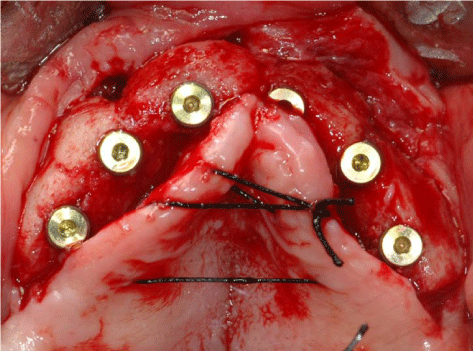
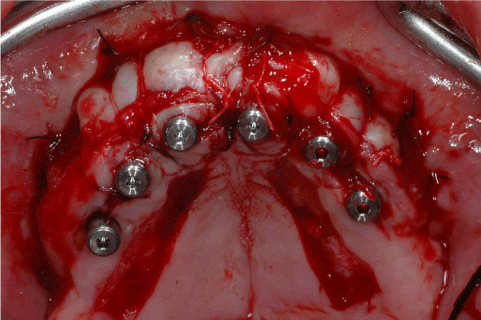
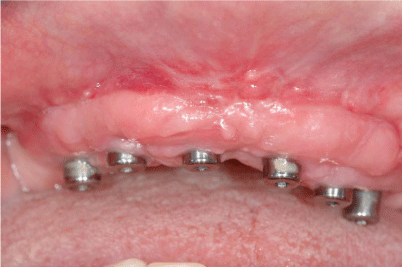
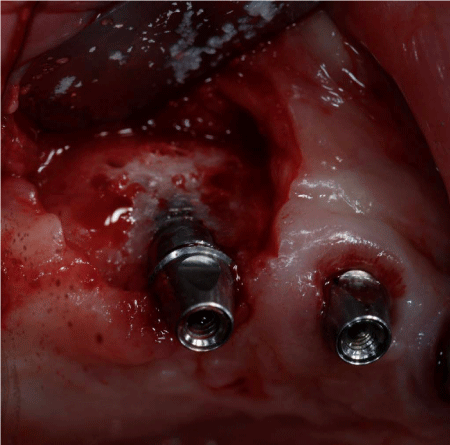
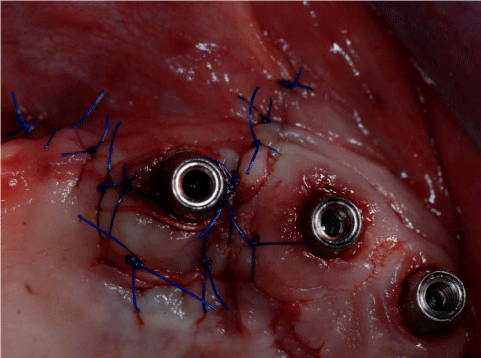
After 8 months from the reconstructive surgery, a CBCT was conducted to assess the graft's integration and therefore to plan the placement of 6 rough-surface implants (Conelog Screw Line 3,8 x 7, Camlog, Switzerland). A radiographic template was required and the surgical procedure was performed under general anesthesia. After elevating a mucoperiosteal flap, the fixation screws were carefully removed and implants were positioned according to the manufacturer instructions in a prosthetically driven position (Figure 10 and 11). A first intention wound closure was obtained. The sutures were removed after 14 days.
Follow-up
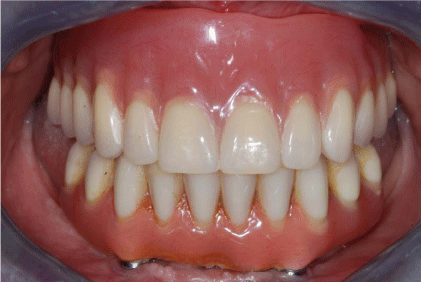
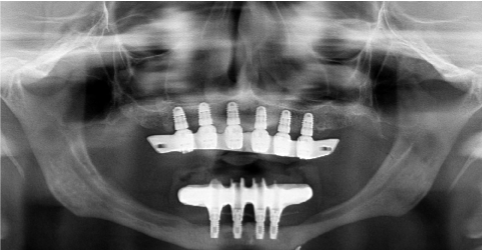
Implants were left unloaded while a removable provisional denture was delivered to the patient with short vestibular flanges in order to avoid graft compression. After 6 months a new surgery was scheduled to obtain implant exposure. A partial thickness flap was elevated and free gingival grafts (harvested from the palate) were performed to increase keratinized mucosa thickness and the correct fornix dimensions was restored thorough a vestibuloplasty (Figure 12 and 13). After 5 months, the definitive overdenture prosthesis was completed and the teeth were articulated on a head to head position (Figure 14 and 15). The patient was included in a strict professional hygiene recall protocol and underwent clinical evaluation every 6 months. A radiological follow-up evaluation with periapical X-rays and orthopantomographs was conducted once a year (Figure 16). After 1 year, in presence of a soft tissue dehiscence in the superior left maxilla (2 x 2 mm), a surgical revision of the wound combined with a coronally advanced flap with epithelial and connective graft was performed (Figure 17-19). After the elevation of a full thickness flap, the lesion was carefully cleaned, and any small sequestered bony fragments were removed. The hygiene procedures are reviewed, and the patient is asked to rinse frequently with chlorhexidine 0.2% in an effort to keep the area moist and clean. After 2 weeks the wound was in good conditions and after several clinical and radiographic controls within three years no more complications were shown (Figure 20).
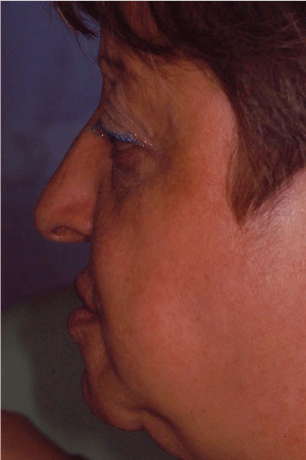
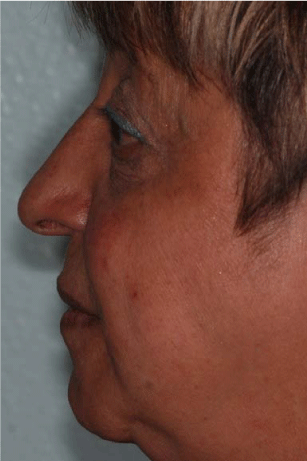
Figure 1
Figure 1
The picture shows the initial exterior profile of the patient. The subsidence of the higher soft perioral tissue is enhanced.
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Figure 7
Allogenic fresh-frozen cortico-cancellous block grafts pre-contoured onto a stereolithographic model.
Figure 8
Figure 9
Figure 10
Figure 10
The total-thick flap raised on second surgery shows the complete osteointegration of the FFB bone grafts.
Figure 11
Figure 12
Discussion
The use of FFB for the reconstruction of atrophic edentulous ridges has been proposed to reduce morbidity related to AB harvesting, in particular when extraoral donor sites, such as the ilum, are used. However, results reported in the literature concerning the use of FFB for the reconstruction of atrophic alveolar ridges are contradictory. Some authors believe that the principal concern regarding allografts is the risk of infectious disease transmission, such as acquired immunodeficiency syndrome [9]. With the standard protocols applied by the bone banks, the risk of viral transmission is virtually nonexistent as it is less than 1:200,000 for hepatitis C virus and 1:1 million for human immunodeficiency virus [10]. The presence of complications related to the use of fresh-frozen bone allografts is well known in the orthopedic literature: a 10-years complication rate from large bone reconstructions variable from 30% to 60% is reported [11]. The primary causes of fresh-frozen bone allografts failure are due to engraftment failure, infection and micro fracture. The structural strength of FFB grafts depends on several factors such as bone mineral density, age and sex of the donor, the graft size and procedures for preservation of FFB. Lumetti et al. [12] underlined that denser grafts showed less resorption than low-density grafts, but cancellous grafts compared to cortical grafts showed a more rapid revascularization. A gradual decrease with time in the biomechanical strength of grafted homologous bone has been demonstrated by De Luiz et al. [13] in their study used computed tomography (CT), histology, and histomorphometry to assess the time-dependent rates of resorption and incorporation of fresh-frozen bone allografts at 4,6 and 8 months after the graft procedure and before implant placement; all of the groups showed remains of grafted tissue at the second stage. The 4-month group showed significantly greater amount of graft remains compared to the 6 ant 8 months groups. Such characteristic can lead to clinical failures and was associated with the presence of microfractures. As neovascularization represents an early event closely associated with bone neoformation, Buffoli et al. [14] found a significative percentage of CD34-positive vessels in the regenerated bone. Spin-Neto et al. [15] made a histological analysis of fresh-frozen onlay bone allografts (ALs), compared with autografts (AT), in patients who needed maxillary reconstruction prior to dental implants placement. Clinically, all grafts were found to be firm in consistency and well-incorporated to the receptor bed. Histological analysis showed a large amount of necrotic bone surrounded by few spots of new-formed bone in the AL group, suggesting low rate of graft remodeling. In the AT group, an advanced stage of bone remodeling was seen. The delayed remodeling of the AL, evidenced in the study by little or no presence of active osteoclasts and osteoblasts, could have direct implications over its clinical use: in particular it could be related to long term complications such as bone exposure. Compared to autologous bone, AL presents the disadvantage that it cannot be freshen, because of its main composition of necrotic bone. Furthermore, Simpson et al. [16] showed the evidence of osteoblast-related vital cells escaping the freezing process during AL preparation; these cells could survive the suggested AATB protocol and might be involved in specific immune responses due to the presence of anti-HLA-specific antibodies against AL grafts, which could have an adverse effect on the graft’s incorporation and increase the incidence of rejection [17]. Indeed, in a recent case series report of similarly performed maxillary alveolar ridge augmentations, a gradual decrease in the amount of necrotic bone from 6 to 9 months post-operatively was observed (61% to 41%, respectively) [18]. It is reasonable to expect that necrotic bone would more readily develop microcracks due to occlusal loading compared to living bone and therefore would also resorb more readily than living bone, because no potential for microcrack repair can be expected in necrotic bone. The clinical impact of the poorer graft incorporation observed for the AL grafts compared with AT on the long-term performance of oral implants placed in AL grafts remains obscure, and no firm assumptions can be made on the basis of the available literature in terms of survival or rate of biological complications. Clinical experience of the authors suggests that better results have been achieved on the superior maxilla rather than on the mandibula, but further long term studies are needed.
Figure 13
Figure 14
Figure 15
Figure 16
Figure 17
Figure 18
Figure 19
Figure 20
Conclusion
In conclusion, the use of homologous FFB grafts has some advantages including: osteoconductive and osteoinductive qualities, reduction of postoperative discomfort for the patient, availability of graft in suitable quantity and quality, reduction of operating time (because the grafts are prepared on stereolithographic models) and less resorption compared to iliac crest autogenous bone [19] . The author’s experience support the hypothesis that fresh-frozen bone allografts can be successful as graft material for the treatment of important maxillary ridge defects. Once adequate surgical techniques are adopted, this type of bone graft can be safely used in regions of implant placement as a suitable alternative to autogenous grafts. Nevertheless further studies are needed to evaluate clinical and histological success in the long term.
References
- Cawood JI, Howell RA. A classification of the edentulous jaws. Int J Oral Maxillofac Surg. 1988; 17: 232-236.
- Pudwill ML, Wentz FM. Microscopic anatomy of edentulous residual alveolar ridges. J Prosthet Dent. 1975; 34: 448-455.
- Nedelman CI, Bernick S. The significance of age changes in human alveolar mucosa and bone. J Prosthet Dent. 1978; 39: 495-501.
- Nkenke E, Radespiel-Tröger M, Wiltfang J, Schultze-Mosgau S, Winkler G, Neukam FW. Morbidity of harvesting of retromolar bone grafts: a prospective study. Clin Oral Implants Res. 2002; 13: 514-521.
- Carini F, Longoni S, Amosso E, Paleari J, Carini S, Porcaro G. Bone augmentation with TiMesh. autologous bone versus autologous bone and bone substitutes. A systematic review. Ann Stomatol (Roma). 2014; 5: 27-36.
- Khan SN, Cammisa FP, Jr., Sandhu HS, Diwan AD, Girardi FP. Lane JM, The biology of bone grafting. The Journal of the American Academy of Orthopaedic Surgeons 2005; 13: 77-86.
- Gazdag AR, Lane JM, Glaser D, Forster RA. Alternatives to autologous bone graft. Efficacy and indications. J Am Acad Orthop Surgz. 1995; 3: 1-8.
- Aho AJ, Eskola J, Ekfors T, Manner I, Kouri T, Hollmen T. Immune responses and clinical outcome of massive human osteoarticular allografts. Clin Orthop Relat Res. 1998; 346: 196-206.
- Buck BE, Resnick L, Shah SM, Malinin TI. Human immunodeficiency virus cultured from bone. Implications for transplantation. Clin Orthop Relat Res. 1990; 251: 249-253.
- Albert A, Leemruse T, Druez V, Delloye C, Cornu O. Are bone autografts still necessary in 2006? A three-year retrospective study of bone grafting. Acta Orthop Belg. 2006; 72: 734-740.
- Wheeler DL. Enneking WF. Allograft bone decreases in strength in vivo over time. Clin Orthop Relat Res. 2005; 435: 36-42.
- Lumetti S, Galli C, Manfredi E, Consolo U, Marchetti C, Ghiacci G, et al. Correlation between density and resorption of fresh-frozen and autogenous bone grafts. Biomed Res Int. 2014; 2014: 508328.
- Deluiz D, Oliveira LS, Pires FR, Tinoco EM. Time-dependent changes in fresh-frozen bone block grafts: tomographic, histologic, and histomorphometric findings. Clin Implant Dent Relat Res. 2015; 17: 296-306.
- Buffoli B, Boninsegna R, Rezzani R, Poli PP, Santoro F, Rodella LF. Histomorphometrical evaluation of fresh frozen bone allografts for alveolar bone reconstruction: preliminary cases comparing femoral head with iliac crest grafts. Clinical Implant Dentistry and Related Research. 2013; 15: 791-798.
- Spin-Neto R, Landazuri Del Barrio RA, Dias Pereira LA, Rosemary Chiérici Marcantonio A, Marcantonio E, Marcantonio E JR. Clinical Similarities and Histological Diversity Comparing Fresh Frozen Onlay Bone Blocks Allografts and Autografts in Human Maxillary Reconstruction. Clinical Implant Dentistry and Related Research, 2013; 15: 490-497.
- Simpson D, Kakarala G, Hampson K, Steele N, Ashton B. Viable cells survive in fresh frozen human bone allografts. Acta Orthopaedica. 2007; 78: 26–30.
- VandeVord PJ, Nasser S, Wooley PH. Immunological responses to bone soluble proteins in recipients of bone allografts. Journal of Orthopaedic Research. 2005; 23: 1059–1064.
- Acocella A, Bertolai R, Ellis E, Nissan J, Sacco R. Maxillary alveolar ridge reconstruction with monocortical fresh-frozen bone blocks: a clinical, histological and histomorphometric study. Journal of Craniomaxillofacial Surgery. 2012; 40: 525–533.
- Maiorana C, Poli P, Borgonovo AE, Rancitelli D, Frigo AC, Pieroni S, et al. Resorbed jaws reconstructed with appositional fresh-frozen bone allografts. Submitted. 2015.