Case Report
Podocyticin Folding Glomerulopathy: Pathological Entity or a Distinct Disease Process
Adil Jadoon1, Paul Killen2 and Puneet Garg1*
1Department of Nephrology, University of Michigan, USA
2Departement of Pathology, University of Michigan, USA
*Corresponding author: Puneet Garg, Division of Nephrology, University of Michigan School of Medicine, 1150 W. Medical Center Drive, MSRB2, Rm 1570D, Ann Arbor, MI 48109, USA
Published: 29 Jul, 2016
Cite this article as: Jadoon A, Killen P, Garg P. Podocyticin
Folding Glomerulopathy: Pathological
Entity or a Distinct Disease Process.
Ann Clin Case Rep. 2016; 1: 1061.
Abstract
We describe a 32-year-old pregnant female with a very distinctive glomerular morphology. She presented with increasing proteinuria in her third trimester and carried a pre-existing diagnosis of systemic lupus erythematosus (SLE). Renal biopsy showed diffusely thickened capillary loops suggestive of class V membranous lupus nephritis. However the immunofluorescence was negative and on electron microscopy the glomerular basement membrane (GBM) was diffusely thickened containing numerous cytoplasmic processes or projections that appear to extend form the podocytes and the endothelial cells which in some locations appear to extend across the full thickness of the GBM. This unique morphology has been exclusively described in patients from Japan and labeled as Podocytic Infolding Glomerulopathy (PIG). The clinicopathological significance of podocyte infolding however has not been fully ascertained and it is unclear if PIG is a morphological entity or a distinct glomerular disease.
Keywords: Podocytic Infolding Glomerulopathy (PIG); Proteinuria; Glomerular disease
Introduction
Podocytic infolding glomerulopathy (PIG) is a rare pathological entity that has only been described in the Japanese population [1-7]. PIG usually presents with proteinuria and light microscopy findings that are similar to membranous nephropathy. Electron micrographs show a distinctive lesion with podocyteinjuryandinfolding into the glomerular basement membrane. Majority of patients have been reported to suffer from a variety of connective tissue diseases particularly SLE [3]. There has also been considerable controversy about whether it represents a transient pathological state in the development of membranous nephropathy or whether this is a new disease entity all together. We report a case of PIG in a young pregnant lupus patient of Asian Indian origin. To our knowledge there is no published case reports of PIG outside of Japan indicating perhaps this condition is more widely distributed and not restricted to the Japanese population.
Case Report
A 32 years old female of Asian Indian ancestry was referred to the glomerular disease clinic
at the University of Michigan with complaints of worsening proteinuria in the third trimester of
her pregnancy. In 2007, she had presented to an outside clinician with fatigue, rash, sacroillitisand
arthralgia’s involving small joints in her hands and feet. She had a positive ANA and elevated
double stranded DNA titers (1:320), establishing a diagnosis of SLE. She responded well to oral
Prednisone with resolution of most of her symptoms but her complements remained low suggestive
of serological disease activity and she was maintained on Prednisone 5-10 mg daily. She became
pregnant in 2014 after in vitro fertilization therapy and at 28 weeks of pregnancy was noted to have
an increase in the urine protein creatinine ratio to 0.8 mg/mg (baseline of 0.2 mg/mg or less) and her
creatinine remained stable at 0.6 mg/dl. Her local physician increased her Prednisone dose to 20 mg
daily and she was referred to the rheumatology clinic at the University of Michigan.
During her visit with rheumatology, the complements were in the normal range (C3: 85, C4:
34) with a mild anemia (Hgb: 9.1) but her urine protein creatinine ratio was however further
elevated at 1.1 mg/mg. This was concerning for a lupus flare or early pre-eclampsia and she was
referred to Nephrology for evaluation and a possible renal biopsy. Her double stranded DNA
was increased at 44.8 (0-7 iu/ml) and he blood pressure was at 120/73 mm Hg, which does not
rule out early pre-eclampsia. She underwent an uncomplicated renal biopsy given the unclear
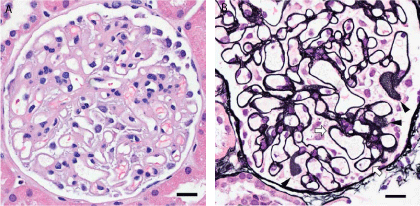
etiology of her worsening proteinuria (SLE activation versus pre-eclampsia). Light microscopy sampled 27 glomeruli, which revealed diffuse thickening of the mesangium with negligible mesangial hypercellularity (Figure 1A). Jones stained sections demonstrated numerous “pinhole” defects in tangential sections of the basement membrane (Figure 1B, arrows) and small sub-epithelial spikes in some vascular loops (Figure 1B, arrowheads). Immunofluorescence staining of 8 glomeruli was negative for IgG, IgA, C1q, C4, C3, fibrinogen, kappa- and lambda-light chains. There was minimal staining of mesangial IgM deposits. Electron microscopy was remarkable for diffuse epithelial cell foot process effacement, microvillous change and occasional electron dense deposits. Glomerular basement membrane (GBM) was markedly thickened (800-1000 nm). Endothelial cells were somewhat swollen with occasional cell processes projecting into the GBM and in rare instances these appeared to traverse the full thickness of the basement membrane (Figure 2, oval). The mesangium was only mildly expanded with no discernible deposits but there were numerous mesangial cellular processes some of which projected into the GBM (Figure 2, arrowheads). This appearance was not suggestive of lupus nephritis but more consistent with PIG. Given the lack of an active inflammatory process on her renal biopsy she was treated conservatively and delivered at term. After delivery her proteinuria resolved within a month (urine protein-creatinine ratio <0.2 mg/mg)and her serum creatinine remained within normal range.
Figure 1
Figure 1
(A): Light microscopy showing diffuse thickening of glomerular basement membrane. (B) Jones stained sections demonstrated numerous “pinhole” defects in tangential sections of the basement membrane (arrowheads) and small sub-epithelial spikes in some vascular loops (arrows).
Figure 2
Figure 2
Transmission Electron Micrographs shows global foot process effacement. Thickened glomerular basement membrane with diffuse distribution of microstructures in the GBM. The invading structures arise from both sides of the glomerular basement membrane (arrowheads) and at places appear to traverse the full thickness of the basement membrane (Bottom right panel, oval).
Discussion
PIG is a histo-pathological entity that has recently been described as perhaps a new disease process in case reports originating exclusively from Japan. The pathological hallmark of this condition is its microscopic appearance. Light microscopy shows a thickened basement membrane with non-argentaffin holes (bubbles/stipples) and occasional spikes [3]. Transmission electronmicroscopy is characterized by vesicular and microtubular structures inside the GBM. These structures have been suggested to represent infolding of podocytes into the GBM [2,8,9].
Joh et al. [3] initially reported these unique morphological findings and proposed the terminology “PodocyticInfoldingGlomerulopathy”. They went on to classify the pathology into three different types: the presence of spikes or podocyte infolding into the GBM alone constitutes type A, type B is characterized by both spikes and intra membranous microstructures in the GBM and type C with only intra membranous structures. The utility of this classification in terms of disease severity or prognosis is however not clear.
The patients present with proteinuria of variable intensity but frequently less than a gram a day, some patients however may have overt Nephrotic syndrome. The majority of patients also have a normal serum creatinine. The patients are young (average age 41.8 years) and females out-number males by a ratio of 3:1. The disease in about 2/3rd of the cases is seen in association with other immunological diseases, most notably SLE and Sjogrens syndrome [5]. There have been case report that have described PIG with a new diagnosis of multiple myeloma [10], hepatitis B virus infection [11] and in a case of vesicoureteral reflux [12] suggesting that PIG perhaps might be a consequence of non-specific podocyte injury.
The pathophysiology of this disease process is still unclear and a large proportion of the reported cases are in association with lupus class V membranous glomerulonephritis or idiopathic membranous nephropathy based on the immunofluorescence pattern, which was not the case in our patient. Podocyte infolding can occasionally be seen in cases of membranous nephropathy [3,13], however there are significant differences in histo-pathological characteristics between PIG and idiopathic membranous nephropathy suggesting a different disease process. In addition some case reports have suggested an association with excessive complement activation and the presence of complement components in the cellular processes but this is not found in all biopsies and some have in fact no complement deposition, raising questions about the possible mechanism of injury [6]
Our case is unique in being the first case of this entity reported outside of Japan and possibly as a direct result of her pregnancy. The patient is similar in age to the cases described in the literature and she had serologically active Lupus for years. Her Lupus was however serologically quiescent at the time of her presentation which raised the possibility of an alternative diagnosis and necessitated a renal biopsy. In addition she did not have evidence of an active immune process on her biopsy, all of which argues against being class V lupus nephritis. Worsening of her proteinuria with pregnancy and resolution after delivery indicates an association, and perhaps the trigger for PIG was hemodynamic changes associated with pregnancy. Her biopsy did however show evidence of diffuse foot process effacement indicating diffuse podocyte injury with resultant infolding. Interestingly our patient also had some evidence of endothelial injury and edema with invasion of the GBM by processes from the endothelium which again could be a consequence of her pregnancy. This may suggest a role for the endothelial cells in this process, which has not been described previously though speculations of an imbalance between basement membrane deposition and resorption by the podocytes have been made. Involvement of endothelial cells would not be unexpected given the role of both endothelial cells and podocytes in formation of the glomerular basement membrane.
In summary we report the first case of PIG in a patient outside of Japan indicating that this disease entity is not limited to a particular population or geography and that it maybe a new disease entity which is just beginning to be recognized. Further investigations into the mechanisms involved would be revealing in regards to the mechanisms that regulate the formation of the highly dynamic glomerular basement membrane.
References
- Joh K, Makino H. Proposal of podocytic infolding glomerulopathy as a new disease entity. Clinical and experimental nephrology. 2008; 12: 417-418.
- Joh K, Taguchi T, Kobayashi Y, Sato H, Nishi S, Katafuchi R, et al. [A preliminary report of national research on podocytic infolding glomerulopathy]. Nihon Jinzo Gakkai shi. 2007; 49: 61-69.
- Joh K, Taguchi T, Shigematsu H, Kobayashi Y, Sato H, Nishi S, et al. Proposal of podocytic infolding glomerulopathy as a new disease entity: a review of 25 cases from nationwide research in Japan. Clinical and experimental nephrology. 2008; 12: 421-431.
- Kitazawa K, Joh K, Akizawa T. A case of lupus nephritis coexisting with podocytic infolding associated with Takayasu's arteritis. Clinical and experimental nephrology. 2008; 12: 462-466.
- Koike K, Utsunomiya Y, Ito Y, Tokudome S, Miyazaki Y, Suzuki T, et al. A case of glomerulopathy showing podocytic infolding in association with Sjogren's syndrome and primary biliary cirrhosis. Clinical and experimental nephrology. 2008; 12: 489-493.
- Tanaka M, Watanabe K, Asahi K, Katoh T, Watanabe T. Lupus nephritis with podocytic infolding and intramembranous microstructures. Clinical and experimental nephrology. 2008; 12: 485-488.
- Yoshimura K, Joh K, Kitamura H, Takahashi Y, Yokote S, Kasai K, et al. A case report of glomerulopathy-associated podocytic infolding in a patient with tumor lysis syndrome. Clinical and experimental nephrology. 2008; 12: 522-526.
- Fujigaki Y, Muranaka Y, Sakakima M, Ohta I, Sakao Y, Fujikura T, et al. Analysis of intra-GBM microstructures in a SLE case with glomerulopathy associated with podocytic infolding. Clinical and experimental nephrology. 2008; 12: 432-439.
- Joh K. Renal Pathology: SY23-2 a case of a new disease entity: podocytic infolding glomerulopathy. Pathology. 2014; 46: S41.
- Harada M, Kamijo Y, Ehara T, Shimojo H, Shigematsu H, Higuchi M. A case of podocytic infolding glomerulopathy with multiple myeloma. BMC nephrology. 2014; 15: 32.
- Nishi S, Imai N, Saito T, Ueno M, Arakawa M, Oota T, et al. A nephropathy presenting the microparticles in the glomerular basement membrane in a patient of hepatitis B viral infection. Clinical and experimental nephrology. 2008; 12: 518-521.
- Matsuo T, Kobayashi Y, Nemoto N, Sano T, Kamata K, Shigematsu H. A nephrotic case of vesicoureteral reflux representing focal segmental glomerulosclerosis associated with podocytic infolding lesions. Clinical and experimental nephrology. 2008; 12: 494-500.
- Masuda Y, Mii A, Shimizu A, Fujita E, Aki K, Ishikawa K, et al. Invagination and infolding of podocytes in glomerular basement membrane in the cases of primary membranous nephropathy. Clinical and experimental nephrology. 2008; 12: 440-449.