Case Report
Report of Two Cases of Accessory Splenic Tissue Mimicking a Neoplasm
Henderson-Jackson EB1,2* and Ghayouri M2
1Departiment of Pathology and Cell Biology, University of South Florida, Tampa FL, USA
2Department of Anatomic Pathology, H. Lee Moffitt Cancer Center, Tampa FL, USA
*Corresponding author: Evita B. Henderson-Jackson, Department of Anatomic Pathology, H. Lee Moffitt Cancer Center, 12902 Magnolia Drive Tampa FL 33612, USA
Published: 10 Jul, 2016
Cite this article as: Henderson-Jackson EB, Ghayouri M.
Report of Two Cases of Accessory
Splenic Tissue Mimicking a Neoplasm.
Ann Clin Case Rep. 2016; 1: 1045.
Abstract
Accessory spleen has been reported to occur in about 10-30% of the population and has been identified in a variety of areas such as the tail of the pancreas, the pelvis and the scrotum; however, the splenic hilum is the most common site. As imaging modalities improve, accessory spleens are frequently detected as an incidental finding on computed tomography (CT) and magnetic resonance imaging (MRI) which may mimic a neoplasm. We report a case of intrapancreatic accessory spleen simulating a neuroendocrine neoplasm and a case of accessory spleen presenting as a submucosal mass suggestive of a gastrointestinal stromal tumor. Finally, we discuss the common differential diagnosis, radiologic features, and possible management of accessory spleens.
Keywords: Splenic tissue; Neoplasm; Neuroendocrine tumor
Introduction
An accessory spleen is an congenital foci of normal splenic tissue separate from the main body
of the spleen [1]. This entity is relatively common. According to autopsy studies, accessory spleens
occur in about 10-30% of the population and in 16% of patients undergoing contrast-enhanced
computed tomography (CT) of the abdomen [2,3]. Accessory splenic tissue have been identified in
areas along the splenic vessels, the tail of the pancreas, in the wall of the jejunum, within intestinal
mesentery, the greater omentum, the pelvis, and the scrotum [4]. However, the splenic hilum is the
most common location for accessory spleens.
Accessory spleens are usually asymptomatic and clinically insignificant, but in some situations
they simulate tumors. Currently, there are no definitive clinical or radiologic criteria for the
differentiation of ectopic splenic tissue from malignancy. Definitive diagnosis is primarily attained
from surgical resection. In this report, we analyze two cases of surgically treated accessory spleens
which radiologically mimicked a neoplasm.
Case Report
Case 1
A 77 year old gentleman with a recent diagnosis of hepatocellular carcinoma (HCC) with
underlying hepatitis C disease was referred to H. Lee Moffitt Cancer Center after surgical resection
of his malignancy for further treatment recommendations. His past medical history was significant
for prostate cancer treated with radiation seed implants and multiple previous surgeries secondary
to a motor vehicle accident and electric bicycle accident. A routine follow-up CT of the chest and
abdomen identified a 2.0 cm mildly hyper vascular solid nodule in the tail of his pancreas. Clinically,
the imaging findings were highly suggestive of a neuroendocrine tumor. The patient underwent an
endoscopic ultrasound (EUS) with fine-needle aspiration (FNA) of the lesion which demonstrated a
hypoechoic mass in the tail of the pancreas corresponding to the CT findings. Unfortunately, the FNA
specimen comprised of only scattered lymphocytes, degenerated cells, and debris. Surgical resection
of the indeterminate lesion was recommended. The patient received a distal pancreatectomy with
splenectomy. Grossly, sections of the pancreas revealed a 1.5 x 1.4 x 1.1 cm accessory spleen. The
remaining pancreatic tissue and spleen was unremarkable. Histologic sections demonstrated an
intra pancreatic accessory spleen (Figure 1) and focal pancreatic intraepithelial neoplasia (PanIN
1b). The intra pancreatic accessory spleen is composed of red pulp, made up of numerous vascular
sinuses, and white pulp, consisting of lymphoid follicles and cells of the reticuloendothelial system.
There was no evidence of malignancy. The patient’s postoperative course was complicated by
bilateral pulmonary embolisms and a persistent fluid collection near his incision site. A month after surgery the fluid collection at the operative site resolved and he remained stable on his Coumadin regimen.
Case 2
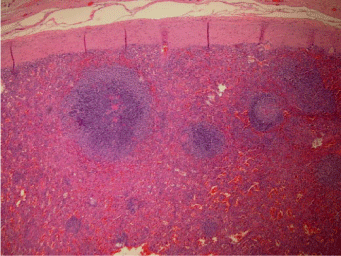
A 49 year-old gentleman with a remote history of multiple injuries secondary to a motor vehicle accident including a ruptured spleen presented to his primary care physician complaining of atypical substernal chest pain. A CT angiogram of his coronary vessels and a nuclear stress test was unremarkable. He received a chest CT as part of the work-up for his chest pain which identified an incidental soft tissue lesion between the left diaphragm and the stomach measuring 2.6 cm in greatest dimension. There was no lymphadenopathy or effusions seen. An esphagogastroduodneoscopy was performed which identified a submucosal lesion in the fundus of the stomach corresponding to the CT findings. There were no infiltrative characteristics, hyervascularity, or surface ulceration seen. Unfortunately, the fundic mucosal biopsy revealed only mild chronic gastritis. At this point, a EUS with FNA of the lesion was recommended. The EUS procedure identified a hypoechoic mass arising from the muscular wall of the stomach. The FNA specimen demonstrated a few spindle cells and capillaries in a background of abundant lymphocytes. A portion of the specimen was sent for flow cytometry studies that revealed a mixed population of polyclonal B lymphocytes and reactive T lymphocytes. In regards to the gastric mass, clinicians suspected a possible gastrointestinal stromal tumor (GIST). However, after two attempts to biopsy the mass resulted in no definite diagnosis, surgical resection was recommended. The patient underwent a partial gastrectomy with a portion of his left diaphragm excised. Gross evaluation of the resection specimen revealed an ovoid, well-defined encapsulated nodule, measuring 1.3 cm in greatest dimension, between the diaphragmatic tissue and gastric wall. Cut surfaces of the nodule were soft, red-brown. Microscopically the nodule was composed of lymphoid follicles and splenic pulp (Figure 2). The gastric mucosa showed chronic gastritis with reactive changes. There was no evidence of malignancy. The findings were compatible with an accessory spleen. The patient tolerated the procedure well without complications.
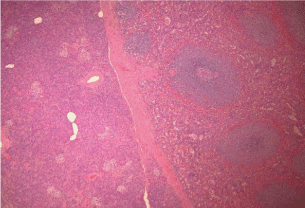
Figure 1
Figure 1
Splenic tissue surrounded by fibrous capsule and adjacent pancreatic parenchyma (Hematoxylin and Eosin staining, 4X).
Figure 2
Discussion
Intrapancreatic accessory spleen has been documented and described in numerous previous case reports [4-19]. On the other hand, accessory spleen masquerading as a gastric submucosal tumor has been rarely reported [20-22]. We report a case of intrapancreatic accessory spleen and a case of an accessory spleen involving the stomach. Since their origin is congential, they are primarily found in areas within the embryologic dorsal mesentery of the stomach and pancreas [23,24]. They are usually asymptomatic as in our cases, but symptoms may arise and have clinical significance in certain situations which include torsion, spontaneous rupture, hemorrhage or causes hematologic disorders such as idiopathic thrombocytopenic purpura [4].
Due to advanced imaging techniques, accessory spleens are often detected incidentally. On sonography, intrapancreatic accessory spleens are well-defined and round or ovoid with a mildly echogenic and homogenous texture identical to that of the main spleen, surrounded by a high-amplitude interface and showing enhancement behind the lesion [7,25,26]. On color or power Doppler sonography images, blood supply to intra pancreatic accessory spleens from splenic artery or vein can be seen in some patients [7]. In contrast-enhanced ultrasonography (US) of intrapancreatic accessory spleens using Levovist, an ultrasound contrast agent containing air micro bubbles, show in homogenous enhancement related to the different flow rate through the cords of the red and white pulp in the arterial phase. In the portal venous phase, intra pancreatic accessory spleens become homogenous. Lastly, during the “hepatosplenic” phase the micro bubbles become trapped within the splenic parenchyma showing prolonged enhancement [27]. In comparison to surrounding pancreatic parenchyma, the intra pancreatic accessory spleen appears to show greater echo enhancement.
There are several characteristic features associated with intra pancreatic accessory spleens on CT and MRI imaging. On CT imaging they are well-defined, round homogenous masses usually smaller than 2 cm in diameter [7]. However, in rare instances accessory splenic tissue may appear hypodense in comparison with surrounding pancreatic tissue and the main spleen because enhancement can be retarded by liver cirrhosis [26]. Pancreatic tumors share similar signal characteristics with that of the spleen on MRI imaging modalities making it difficult to differentiate an intra pancreatic accessory spleen from a pancreatic tumor [26]. Interestingly, octreotide scintigraphy used to show the expression of somatostatin receptors in neuroendocrine neoplasms demonstrates somatostatin receptor expression in splenic tissue also [18]. However, super paramagnetic iron oxides (SPIO)-enhanced MRI technique demonstrate similar degree of signal drop in intra pancreatic accessory spleens as compared to native spleen [28]. Similar to technetium-99m heat-damaged red blood cells (HDRBC) scintigraphy in which injected HDRBCs are trapped by splenic tissue, SPIO-enhanced MRI requires the administration of SPIO particles intravenously to be phagocytosed by macrophages of the reticuloendothelial system in the spleen [28]. Although technetium-99m HDRBC scintigraphy is highly specific for detecting splenic tissue, the visualization of minute functioning splenic tissue is difficult. Furthermore, MRI imaging offers superior spatial resolution compared to scintigraphy [26].
The imaging findings in the cases presented in this report were not characteristic of accessory splenic tissue. The patient with the intra pancreatic accessory spleen had CT imaging findings demonstrating mild hypervascularity which can be associated with pancreatic endocrine tumors. In the evaluation of intra pancreatic masses with hypervascularity the differential would include pancreatic neuroendocrine tumors as well as lymphomas, hypervascular metastases, solid pseudopapillary tumors and pancreatic adenocarcinomas. However, benign entities should not be disregarded like intra pancreatic accessory spleen or even lymphoepithelial cyst [4,13]. The patient with the gastric submucosal mass had CT imaging findings suggestive of a GIST. Differential diagnosis for submuocosal gastric masses may include leiomyoma, schwannoma, fibromatosis, inflammatory myofibroblastic tumor, neuroendocrine tumor, leiomyosarcoma, and more. But an accessory spleen should be included in the differential. Therefore, a clear-cut distinction between accessory splenic tissue and a neoplasm/malignancy is extremely important when considering the possible differential but, is hard to attain with current technology.
EUS-guided FNA may provide a reliable diagnosis, thus avoiding unnecessary surgery. Schreiner et al. [29] reported the first series of intra pancreatic accessory spleen diagnosed by EUS-guided FNA. The cytologic features were nonspecific and included predominantly small lymphocytes with a subset of histiocytes, eosinophils, and plasma cells. Thin-walled blood vessels may also be seen, representing splenic sinuses. Interestingly, endothelial cells lining splenic sinuses are CD8 positive whereas systemic endothelial cells and hemangioma are negative [29]. There is the potential to diagnose accessory splenic tissue without resulting in surgery. Unfortunately, cytologic specimens may be suboptimal or insufficient as seen in our cases. Surgery in certain situations may be the best method for diagnosis and provide peace of mind to patients, in that; they are able to know definitively that their mass is benign.
In conclusion, modern imaging modalities will likely lead to an increase number of accessory spleens being discovered which will pose a diagnostic challenge. When an asymptomatic mass is detected involving the embryologic region of which ectopic splenic tissue may arise, an accessory spleen should be within the differential as well as a neoplasm/malignancy. Non-invasive diagnostic techniques are required including imaging with possible biopsy (either FNA or core needle biopsy). Hopefully, the diagnosis of an accessory spleen may be obtained and obviate unnecessary surgery decreasing overall patient morbidity. However, when a tissue diagnosis cannot be obtained from a biopsy, imaging findings are not conclusive, and the clinical suspicion for a neoplasm is high surgical resection is warranted.
References
- Freeman J, Jafri S, Roberts J, Mezwa D, Shirkhoda A. CT of congenital and acquired abnormalities of the spleen. Radiographics. 1993; 13: 597-610.
- Halpart B, Alden Z. Accessory spleens in or at the tail of the pancreas: a survey of 2,700 additional necropsies. Arch Pathol. 1964; 77: 652-654.
- Halpart B, Gyorkey F. Lesions observed in accessory spleens of 311 patients. Am J Clin Pathol. 1959; 32: 165-168.
- Arkadooulos N, Athanasopoulos P, Stafyla V, Karakatsanis A, Koutoulidis V, Theodosopoulos T, et al. Intrapancreatic accessory spleen issues: diagnostic and therapeutic challenges. J Pancreas. 2009; 10: 400-405.
- Meiler R, Dietl K, Novak K, Patzel C. Intrapancreatic accessory spleen. Int Surg. 2010; 95: 183-187.
- Kavic S, Park A. Intrapancreatic accessory spleen: deficiency in diagnosis or therapeutic success? J Gastrointest Surg. 2009; 13: 396.
- Guo W, Han W, Liu J, Jin L, Li J, Zhang Z, et al. Intrapancreatic accessory spleen: a case report and review of the literature. World J Gastroenterol. 2009; 15: 1141-1143.
- Toure L, Bedard J, Sawan B, Mosimann F. Intrapancreatic accessory spleen mimicking a pancreatic endocrine tumor. Can J Surg. 2010; 53: E1-E2.
- Kurmann A, Michel J, Stauffer E, Egger B. Intrapancreatic accessory spleen misdiagnosed as a nonsecreting endocrine tumor: case report and review of the literature. CAse Rep Gastroenterol. 2010; 4: 210-214.
- Hamada T, Isaji S, Mizuno S, Tabata M, Yamagiwa K, Yokoi H, et al. Laparoscopic spleen-preserving pancreatic tail resection for an intrapancreatic accessory spleen mimicking a nonfunctioning endocrine tumor: report of a case. Surg Today. 2004; 34: 878-881.
- Sica G, Reed M. Case 27: Intrapancreatic accessory spleen. Radiology. 2000; 217: 134-137.
- Schwartz T, Sterkel B, Meyer-Rochow G, Gifford A, Samara J, Sywak M, et al. Accessory spleen masquerading as a pancreatic neoplasm. The American Journal of Surgery. 2009; 197: e61-e63.
- Uchiyama S, Chijiiwa K, Hiyoshi M, Ohuchida J, Imamura N, Nagano M, et al. Intrapancreatic accessory spleen mimicking endocrine tumor of the pancreas: case report and review of the literature. J Gastrointest Surg. 2008; 12: 1471-1473.
- Harris G, Kase D, Bradnock H, Mckinley M. Accessory spleen causing a mass in the tail of the pancreas: MR imaging findings. AJR. 1994; 163: 1120-1121.
- Lauffer J, Baer H, Maurer C, Wagner M, Zimmermann A, Buchler M. Intrapancreatic accessory spleen: a rare cause of a pancreatic mass. International Journal of Pancreatology. 1999; 25: 65-68.
- Katzka D, Jaffe D. Clinical Challenges and Images in GI. Gastroenterology. 2006; 131: 350-351.
- Churei H, Inoue H, Nakajo M. Intrapancreatic accessory spleen: case report. Abdom Imaging. 1998; 23: 191-193.
- Brasca L, Zanello A, De Gaspari A, De Cobelli F, Zerbi A, Fazio F, et al. Intrapancreatic accessory spleen mimicking a neuroendocrine tumor: magnetic resonance findings and possible diagnostic role of different nuclear medicine tests. Eur Radiol. 2004; 14: 1322-1323.
- Takayama T, Shimada K, Inoue H, Wakao F, Yamamoto J, Kosuge T. Intrapancreatic accessory spleen. The Lancet. 1994; 344: 957-958.
- Chin S, Isomoto H, Mizuta Y, Wen C, Shikuwa S, Kohno S. Enlarged accessory spleen presenting stomach submucosal tumor. World J Gastroenterol. 2007; 13: 1752-1754.
- Hargrove M, Kilpatrick Z. Pseudotumor of the gastric fundus caused by an accessory spleen. J Louisiana State M Soc. 1969; 121: 386-387.
- Crawford A. Accessory splenic stomach wall abscess mass simulating neoplasm. Aust NZ J Surg. 1974; 44: 39-42.
- Dodds WJ, Taylor A, Erickson S, Stewart E, Lawson T. Radiologic imaging of splenic anomalies. AJR Am J Roentgenol. 1990; 155: 805-810.
- White JD, West A, Priebat D. Splenosis mimicking an intrabdominal malignancy. AM J Med. 1989; 87: 687-690.
- Subramanyam B, Balthazar E, Horii S. Sonography of the accessory spleen. AJR Am J Roentgenol. 1984; 143: 47-49.
- Kim S, Lee J, Han J, Lee J, Kim K, Cho K, et al. Intrapancreatic accessory spleen: findings on MR imaging, CT, US and scintigraphy and the pathologic analysis. Korean J Radiol. 2008; 9: 162-174.
- Kim S, Lee J, Lee J, Han J, Choi B. Contrast-enhanced sonography of intrapancreatic accessory spleen in six patients. AJR Am J Roentgenol. 2007; 188: 422-428.
- Kim S, Lee J, Han J, Lee J, Kang W, Jang J, et al. MDCT and superparamagnetic iron oxide (SPIO)-enhanced MR findings of intrapancreatic accessory spleen in seven patients. Eur Radiol. 2006; 16: 1887-1897.
- Schreiner A, Mansoor A, Faigel D, Morgan T. Intrapancreatic accessory spleen: mimic of pancreatic endocrine tumor diagnosed by endoscopic ultrasound-guided fine-needle aspiration biopsy. Diagn Cytopathol. 2008; 36: 262-265.